Difference between revisions of "AY Honors/Soap Craft/Answer Key 2"
(Fixed some grammatical issues.) |
|||
| Line 22: | Line 22: | ||
Soap is a product used in conjunction with water for cleansing and lubricating. In a domestic setting soaps are usually used for washing, bathing, and other types of housekeeping. In industry soaps are used as thickeners, lubricants, or as part a process before a catalyst is applied. | Soap is a product used in conjunction with water for cleansing and lubricating. In a domestic setting soaps are usually used for washing, bathing, and other types of housekeeping. In industry soaps are used as thickeners, lubricants, or as part a process before a catalyst is applied. | ||
| − | ==2. What three ingredients are | + | ==2. What three ingredients are necessary to make soap?== <!--T:4--> |
<!--T:29--> | <!--T:29--> | ||
| Line 36: | Line 36: | ||
<!--T:6--> | <!--T:6--> | ||
| − | Soaps are made from natural ingredients and detergents are made from man made derivatives. Soap needs to be rinsed in clear water or it will leave a film. Detergents do not leave any residue. Soaps need '''warm'''water to work. Detergents work well in any water temperature. [https://www.nycoproducts.com/resources/blog/simple-science-the-difference-between-soap-and-detergent/] | + | Soaps are made from natural ingredients and detergents are made from man-made derivatives. Soap needs to be rinsed in clear water or it will leave a film. Detergents do not leave any residue. Soaps need '''warm'''water to work. Detergents work well in any water temperature. [https://www.nycoproducts.com/resources/blog/simple-science-the-difference-between-soap-and-detergent/] |
* A [[W:soap|soap]] is a natural fatty substance (called fatty acids) which has been reacted with lye (sodium hydroxide). | * A [[W:soap|soap]] is a natural fatty substance (called fatty acids) which has been reacted with lye (sodium hydroxide). | ||
* A [[W:detergent|detergent]] is any type of cleaner which does not contain a soap as its main ingredient. | * A [[W:detergent|detergent]] is any type of cleaner which does not contain a soap as its main ingredient. | ||
| Line 43: | Line 43: | ||
<!--T:30--> | <!--T:30--> | ||
| − | Toilet soaps are used in domestic settings. " | + | Toilet soaps are used in domestic settings. Usually, what most people refer to as "soap" is technically called a toilet soap, used for household and personal cleaning. |
Non-toilet soaps are components of many lubricating greases and thickeners. Greases are usually mixtures of calcium soap or lithium soap and mineral oil. There are many any other metallic soaps, such as aluminum or sodium. These soaps are used as thickeners to increase the viscosity of oils. | Non-toilet soaps are components of many lubricating greases and thickeners. Greases are usually mixtures of calcium soap or lithium soap and mineral oil. There are many any other metallic soaps, such as aluminum or sodium. These soaps are used as thickeners to increase the viscosity of oils. | ||
| Line 50: | Line 50: | ||
<!--T:31--> | <!--T:31--> | ||
| − | They are used as a lubricating agents in grease and as | + | They are used as a lubricating agents in grease and as thickeners. Artist paints use them as a thickener controlling the flow of the paint. |
| − | Non-toilet lithium soaps are used as components of lithium grease (white lithium)used as a form-release | + | Non-toilet lithium soaps are used as components of lithium grease (white lithium) used as a form-release agent at relatively high temperatures. |
Aluminum soap is used chiefly in lubricating greases, in protective coatings, and in waterproofing compositions. | Aluminum soap is used chiefly in lubricating greases, in protective coatings, and in waterproofing compositions. | ||
| Line 57: | Line 57: | ||
<!--T:32--> | <!--T:32--> | ||
| − | The structure of soap molecules enables them to remove dirt with ease. They consist of a hydrocarbon chain | + | The structure of soap molecules enables them to remove dirt with ease. They consist of a hydrocarbon chain with a sodium or potassium atom at the end. The hydrocarbon end is attracted to oil and repels water, whereas the other end attracts water. When you wash your hands, oily dirt particles are surrounded by soap molecules with their water-loving heads facing outwards. This breaks up the dirt and lets it wash away in the water. [https://www.sciencefocus.com/science/how-does-soap-work/] |
==7. Research the history and origin of soap. Answer the following questions:== <!--T:10--> | ==7. Research the history and origin of soap. Answer the following questions:== <!--T:10--> | ||
| Line 67: | Line 67: | ||
<!--T:34--> | <!--T:34--> | ||
| − | Soap making was known as early as | + | Soap making was known as early as 2800 B.C. [[http://wiki.pathfindersonline.org/w/Adventist_Youth_Honors_Answer_Book/Arts_and_Crafts/Soap_Craft_(General_Conference) ]] |
===b. In what context is soap mentioned in the Bible?=== <!--T:12--> | ===b. In what context is soap mentioned in the Bible?=== <!--T:12--> | ||
| Line 87: | Line 87: | ||
<!--T:17--> | <!--T:17--> | ||
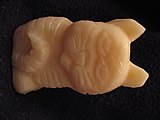
| − | Here are some | + | Here are some Pathfinder soap carvings for examples. |
<!--T:18--> | <!--T:18--> | ||
| Line 96: | Line 96: | ||
<!--T:19--> | <!--T:19--> | ||
| − | You can buy | + | You can buy multi-packs of soap at reasonable prices at large "warehouse" type stores such as Costco or Sam's Club or at large retailers such as Target or Walmart. Ivory brand soap works pretty well for this, though you may wish to check that none of your Pathfinders are allergic to it before you buy it. |
<!--T:20--> | <!--T:20--> | ||
Revision as of 17:00, 26 October 2020
1. What is soap?
Soap is a product used in conjunction with water for cleansing and lubricating. In a domestic setting soaps are usually used for washing, bathing, and other types of housekeeping. In industry soaps are used as thickeners, lubricants, or as part a process before a catalyst is applied.
2. What three ingredients are necessary to make soap?
An alkali, a fat, and water.
Alkali includes lye and potash. Lye makes a harder soap while potash makes a softer soap.
A variety of fats can be used, such as lard, coconut oil, or palm oil.
Water or a substance containing water, such as milks, fruit juices, etc. are also necessary.
3. What is the difference between soap and detergent?
Soaps are made from natural ingredients and detergents are made from man-made derivatives. Soap needs to be rinsed in clear water or it will leave a film. Detergents do not leave any residue. Soaps need warmwater to work. Detergents work well in any water temperature. [1]
- A soap is a natural fatty substance (called fatty acids) which has been reacted with lye (sodium hydroxide).
- A detergent is any type of cleaner which does not contain a soap as its main ingredient.
4. What is the difference in toilet soaps and non-toilet soaps?
Toilet soaps are used in domestic settings. Usually, what most people refer to as "soap" is technically called a toilet soap, used for household and personal cleaning.
Non-toilet soaps are components of many lubricating greases and thickeners. Greases are usually mixtures of calcium soap or lithium soap and mineral oil. There are many any other metallic soaps, such as aluminum or sodium. These soaps are used as thickeners to increase the viscosity of oils.
5. Name three uses of non-toilet soaps.
They are used as a lubricating agents in grease and as thickeners. Artist paints use them as a thickener controlling the flow of the paint. Non-toilet lithium soaps are used as components of lithium grease (white lithium) used as a form-release agent at relatively high temperatures. Aluminum soap is used chiefly in lubricating greases, in protective coatings, and in waterproofing compositions.
6. Discuss how toilet soaps work to clean.
The structure of soap molecules enables them to remove dirt with ease. They consist of a hydrocarbon chain with a sodium or potassium atom at the end. The hydrocarbon end is attracted to oil and repels water, whereas the other end attracts water. When you wash your hands, oily dirt particles are surrounded by soap molecules with their water-loving heads facing outwards. This breaks up the dirt and lets it wash away in the water. [2]
7. Research the history and origin of soap. Answer the following questions:
See the history in the General Conference version of the Soap Craft Honor. [[3]]
a. What is the oldest record of soap?
Soap making was known as early as 2800 B.C. [[4]]
b. In what context is soap mentioned in the Bible?
It is mentioned in Jeremiah 2:22 in the context that soap cannot wash away the stain of our guilt. In Malachi 3:2, soap is mentioned in the context of the Lord's coming. At that time Jesus will be a cleansing agent like a laundry soap. [5]
c. How did WWI affect soap production?
The chemistry of soap manufacturing stayed essentially the same until 1916. During World War I and again in World War II, there was a shortage of animal and vegetable fats and oils that were used in making soap. Chemists had to use other raw materials instead, which were “synthesized” into chemicals with similar properties. These are what are known today as “detergents.” [6]
8. What is soap scum?
Scum is the substance which results from the reaction of the hardness mineral in water with common household soaps. Evident as a bath tub ring or on shower doors.
9. Carve an object from a bar of soap.
Here are some Pathfinder soap carvings for examples.
You can buy multi-packs of soap at reasonable prices at large "warehouse" type stores such as Costco or Sam's Club or at large retailers such as Target or Walmart. Ivory brand soap works pretty well for this, though you may wish to check that none of your Pathfinders are allergic to it before you buy it.
Start by tracing the design onto the bar of soap, etching the lines lightly into the soap with a pointed stick or the tip of a knife. Instruct your Pathfinders on knife safety before handing out the knives:
- Do not draw the knife towards yourself - cut away from your body.
- Pick up a knife by its handle
- Be aware that folding knives can close when under pressure.
- Offer a knife's handle to another person when handing it to another person.
Once the design is etched into the soap, begin removing the soap that does not belong there. Do not remove huge chunks all at once or the soap may break. You can also use the knife to drill holes in the soap.
10. Decorate a bar of soap for a gift.
Soap can be decorated by attaching ribbons with pins. You can also use pins to attach photos (crop them first), pictures cut from magazines, buttons, doilies, etc. Be creative! Pay attention to the colors of the materials you attach to the soap compared to the color of the soap itself. Soap need not be carved before decorating, but it may help to cut any lettering (such as the brand's logo) off before attaching pictures.
If you have pictures to contribute, we'd love to add them to this chapter. Just click the "talk" link at the top of the page!
References