Difference between revisions of "AY Honors/Chemistry/Answer Key"
(+ translate tags) |
(Marked this version for translation) |
||
| Line 1: | Line 1: | ||
<languages /><br /> | <languages /><br /> | ||
| − | <noinclude><translate></noinclude> | + | <noinclude><translate><!--T:1--> |
| + | </noinclude> | ||
{{honor_desc | {{honor_desc | ||
|stage=100 | |stage=100 | ||
| Line 10: | Line 11: | ||
|insignia=Chemistry_Honor.png}} | |insignia=Chemistry_Honor.png}} | ||
| − | ==1. Define the following terms: == | + | ==1. Define the following terms: == <!--T:2--> |
===a. Elements === | ===a. Elements === | ||
| + | <!--T:3--> | ||
A class of substances that cannot be separated into simpler substances by chemical means. | A class of substances that cannot be separated into simpler substances by chemical means. | ||
| − | ===b. Compounds === | + | ===b. Compounds === <!--T:4--> |
| + | <!--T:5--> | ||
A uniform substance composed of two or more elements. | A uniform substance composed of two or more elements. | ||
| − | ===c. Chemical symbols === | + | ===c. Chemical symbols === <!--T:6--> |
| + | <!--T:7--> | ||
Every element is represented using an abbreviation of one or two characters that represent the name of the element. The first character is always capitalized, and the second character if it exists is always lower case. | Every element is represented using an abbreviation of one or two characters that represent the name of the element. The first character is always capitalized, and the second character if it exists is always lower case. | ||
:H represents hydrogen | :H represents hydrogen | ||
| Line 27: | Line 31: | ||
:C represents Carbon | :C represents Carbon | ||
| + | <!--T:8--> | ||
Some of the symbols do not represent the modern name, but an original name. | Some of the symbols do not represent the modern name, but an original name. | ||
:Ag represents argentum (''Latin'') or silver | :Ag represents argentum (''Latin'') or silver | ||
| Line 32: | Line 37: | ||
:W represents wolfram (''German'') or tungsten | :W represents wolfram (''German'') or tungsten | ||
| + | <!--T:9--> | ||
There are 94 elements that are found naturally on earth, and there are another 17 that have been created in atomic accelerators, and have very short half lives. A further 7 elements have been reported but they are still in the confirming/naming stage. All of these elements are organized using a "Periodic Table of Elements" which was created by Dimitri Mendeleev. | There are 94 elements that are found naturally on earth, and there are another 17 that have been created in atomic accelerators, and have very short half lives. A further 7 elements have been reported but they are still in the confirming/naming stage. All of these elements are organized using a "Periodic Table of Elements" which was created by Dimitri Mendeleev. | ||
| − | ===d. Solutions === | + | ===d. Solutions === <!--T:10--> |
| + | <!--T:11--> | ||
A mixture of two or more substances that do not react chemically. If you dissolve sugar, salt, or another substance in water, you are creating a solution. | A mixture of two or more substances that do not react chemically. If you dissolve sugar, salt, or another substance in water, you are creating a solution. | ||
| − | ===e. Atoms === | + | ===e. Atoms === <!--T:12--> |
| + | <!--T:13--> | ||
Atoms are basic building blocks of matter. Atoms range in size from .5Å to 24Å. ( Å is the symbol for a unit of measure known as an angstrom. A blond hair is between 170,000 and 500,000Å in diameter and a black hair is between 560,000 and 1,810,000Å in diameter) | Atoms are basic building blocks of matter. Atoms range in size from .5Å to 24Å. ( Å is the symbol for a unit of measure known as an angstrom. A blond hair is between 170,000 and 500,000Å in diameter and a black hair is between 560,000 and 1,810,000Å in diameter) | ||
| + | <!--T:14--> | ||
Atoms are mostly empty space, but because the electrons are moving so rapidly, the matter we touch can feel very solid. Hydrogen is the simplest atom, it is made of one proton and one electron. A classical model of an atom is to think about the electron orbiting the central proton. If we created a model of this atom with the proton the size of a basket ball, then the electron would be the size of a grain of salt and will be orbiting at a distance of 40,000 feet. | Atoms are mostly empty space, but because the electrons are moving so rapidly, the matter we touch can feel very solid. Hydrogen is the simplest atom, it is made of one proton and one electron. A classical model of an atom is to think about the electron orbiting the central proton. If we created a model of this atom with the proton the size of a basket ball, then the electron would be the size of a grain of salt and will be orbiting at a distance of 40,000 feet. | ||
| + | <!--T:15--> | ||
We cannot directly see atoms with a regular microscope. Light has a wavelength of between 4,000Å and 7,000Å which is about 1000 times the diameter of an atom. Because the wavelength of light is so long it has almost no interaction with the atom. The center of an Atom can be "viewed" and the positions of the electron cloud around an atom can be determined by using an X-ray, (neutron, or electron) beam (which have much smaller wavelengths than light) in such techniques as | We cannot directly see atoms with a regular microscope. Light has a wavelength of between 4,000Å and 7,000Å which is about 1000 times the diameter of an atom. Because the wavelength of light is so long it has almost no interaction with the atom. The center of an Atom can be "viewed" and the positions of the electron cloud around an atom can be determined by using an X-ray, (neutron, or electron) beam (which have much smaller wavelengths than light) in such techniques as | ||
[[w:X-ray_crystallography|X-ray crystallography]], [[w:Neutron_crystallography|Neutron crystallography]], or [[w:Electron_crystallography|Electron crystallography]]. Or a [[w:Scanning_tunneling_microscope|scanning tunneling microscope]] can be used. | [[w:X-ray_crystallography|X-ray crystallography]], [[w:Neutron_crystallography|Neutron crystallography]], or [[w:Electron_crystallography|Electron crystallography]]. Or a [[w:Scanning_tunneling_microscope|scanning tunneling microscope]] can be used. | ||
| − | ===f. Molecules === | + | ===f. Molecules === <!--T:16--> |
| + | <!--T:17--> | ||
Two or more atoms that have bonded to each other. | Two or more atoms that have bonded to each other. | ||
| − | ===g. Periodic Table=== | + | ===g. Periodic Table=== <!--T:18--> |
| + | <!--T:19--> | ||
The periodic table of the chemical elements is a tabular method of displaying the chemical elements. The table illustrates recurring ("periodic") trends in the properties of the elements. | The periodic table of the chemical elements is a tabular method of displaying the chemical elements. The table illustrates recurring ("periodic") trends in the properties of the elements. | ||
{{Small Periodic table}} | {{Small Periodic table}} | ||
| − | ===h. Combustion === | + | ===h. Combustion === <!--T:20--> |
| + | <!--T:21--> | ||
We often use the word combustion to describe the chemical process of a fuel combining rapidly with a oxidizer (usually oxygen)i.e. burning. This process usually is associated with flames, light, heat, and smoke. | We often use the word combustion to describe the chemical process of a fuel combining rapidly with a oxidizer (usually oxygen)i.e. burning. This process usually is associated with flames, light, heat, and smoke. | ||
| − | === i. Acid=== | + | === i. Acid=== <!--T:22--> |
| + | <!--T:23--> | ||
The word acid is from the Latin word ''acidus'' which means sour. In the early days of chemistry, it was a common practice to taste or smell things and record the sensation. Many things that are acidic are sour. Lemon juice is sour because of the citric acid in it. | The word acid is from the Latin word ''acidus'' which means sour. In the early days of chemistry, it was a common practice to taste or smell things and record the sensation. Many things that are acidic are sour. Lemon juice is sour because of the citric acid in it. | ||
| + | <!--T:24--> | ||
Because many substances can be toxic, this probably killed a number of chemists including the famous scientist, Humphry Davy, the man who came up with the theory that explained the nature of an acid. | Because many substances can be toxic, this probably killed a number of chemists including the famous scientist, Humphry Davy, the man who came up with the theory that explained the nature of an acid. | ||
| + | <!--T:25--> | ||
Davy's hypothesis was that acids were substances that contained replaceable hydrogen. This hydrogen could be replaced by metals and this reaction would leave behind a salt. | Davy's hypothesis was that acids were substances that contained replaceable hydrogen. This hydrogen could be replaced by metals and this reaction would leave behind a salt. | ||
| + | <!--T:26--> | ||
:2HCl + Zn → ZnCl<sub>2</sub> + H<sub>2</sub> <br> | :2HCl + Zn → ZnCl<sub>2</sub> + H<sub>2</sub> <br> | ||
:Fe + H<sub>2</sub>SO<sub>4</sub> → H<sub>2</sub> + FeSO<sub>4</sub> | :Fe + H<sub>2</sub>SO<sub>4</sub> → H<sub>2</sub> + FeSO<sub>4</sub> | ||
| − | ===j. Salt=== | + | ===j. Salt=== <!--T:27--> |
| + | <!--T:28--> | ||
A salt is a term for the solid form of an ionic compound, such as Sodium Chloride (Na<sup>+</sup>Cl<sup>-</sup>) or potassium hydroxide (K<sup>+</sup>(OH)<sup>-</sup>). | A salt is a term for the solid form of an ionic compound, such as Sodium Chloride (Na<sup>+</sup>Cl<sup>-</sup>) or potassium hydroxide (K<sup>+</sup>(OH)<sup>-</sup>). | ||
| − | ===k. Proton=== | + | ===k. Proton=== <!--T:29--> |
| + | <!--T:30--> | ||
A positively charged particle that is a part of the nucleus of an atom. Protons have a mass of <math>1.67 \times 10^{-27}</math> kg. One cubic centimeter of water contains <math>6.02 \times 10^{24}</math>protons that form the nucleus of the atoms of hydrogen and oxygen. Hydrogen has one proton at its center and oxygen has 8 protons. | A positively charged particle that is a part of the nucleus of an atom. Protons have a mass of <math>1.67 \times 10^{-27}</math> kg. One cubic centimeter of water contains <math>6.02 \times 10^{24}</math>protons that form the nucleus of the atoms of hydrogen and oxygen. Hydrogen has one proton at its center and oxygen has 8 protons. | ||
| − | ===l. Neutron === | + | ===l. Neutron === <!--T:31--> |
| + | <!--T:32--> | ||
A neutrally charged particle that is a part of the nucleus of an atom. Neutron's have a mass of <math>1.67 \times 10^{-27}</math> kg. When we look at water we find that it is made up of Hydrogen and Oxygen. Hydrogen has no neutrons, but each atom of oxygen has 8 neutrons. | A neutrally charged particle that is a part of the nucleus of an atom. Neutron's have a mass of <math>1.67 \times 10^{-27}</math> kg. When we look at water we find that it is made up of Hydrogen and Oxygen. Hydrogen has no neutrons, but each atom of oxygen has 8 neutrons. | ||
| + | <!--T:33--> | ||
Neutrons are held together by what is called the "Weak Force". Free [[W:Neutron_decay|neutrons decay]] with a half life of about 10.3 minutes into a proton, electron and an electron nutrino. In the neucleus of an atom, the neutrons can be quite stable because of another force known as the "Strong Force" | Neutrons are held together by what is called the "Weak Force". Free [[W:Neutron_decay|neutrons decay]] with a half life of about 10.3 minutes into a proton, electron and an electron nutrino. In the neucleus of an atom, the neutrons can be quite stable because of another force known as the "Strong Force" | ||
| − | ===m. Electron=== | + | ===m. Electron=== <!--T:34--> |
| + | <!--T:35--> | ||
A negatively charged particle with a mass of <math>9.11 \times 10^{-31}</math> kg. Electrons form a cloud about the positively charged nucleus of an atom. | A negatively charged particle with a mass of <math>9.11 \times 10^{-31}</math> kg. Electrons form a cloud about the positively charged nucleus of an atom. | ||
| − | ===n. Distillation === | + | ===n. Distillation === <!--T:36--> |
Distillation, which is heating the liquid to boiling and collecting the condensed vapors, is a useful technique to separate components based on different boiling points. Distillation separates two volatile liquids where the more volatile component is more pure. The farther apart the boiling temperatures, the more likely that the distillation will be successful. The lower the boiling point, the more volatile the compound is and therefore the faster it will vaporize. Success also depends on the interactions between particles, since two dissimilar liquids that are dissolved in each other are likely to form '''azeotropes'''. If this occurs, the liquid and gas phase will both have the same composition and therefore boiling will not cause further separation. | Distillation, which is heating the liquid to boiling and collecting the condensed vapors, is a useful technique to separate components based on different boiling points. Distillation separates two volatile liquids where the more volatile component is more pure. The farther apart the boiling temperatures, the more likely that the distillation will be successful. The lower the boiling point, the more volatile the compound is and therefore the faster it will vaporize. Success also depends on the interactions between particles, since two dissimilar liquids that are dissolved in each other are likely to form '''azeotropes'''. If this occurs, the liquid and gas phase will both have the same composition and therefore boiling will not cause further separation. | ||
| − | ===o. Fractional Distillation=== | + | ===o. Fractional Distillation=== <!--T:37--> |
[[File:Fractional distillation lab apparatus.svg|thumb|350px|Fractional distillation aparatus]] | [[File:Fractional distillation lab apparatus.svg|thumb|350px|Fractional distillation aparatus]] | ||
Fractional distillation is a technique in which several distillations occur in the same column, mediated by some sort of porous medium. It allows the separation of substances with closer boiling points than simple distillation, and also makes the distillation of the same solutions more efficient. Fractional distillation yields better separation in comparison to simple distillation due to few factors. One is that the distillation column is usually packed with copper, stool wire or glass beads. This increases the surface area, which enables efficient and continuous vaporization and condensation by exchanging heat between the vapor and the condensate. | Fractional distillation is a technique in which several distillations occur in the same column, mediated by some sort of porous medium. It allows the separation of substances with closer boiling points than simple distillation, and also makes the distillation of the same solutions more efficient. Fractional distillation yields better separation in comparison to simple distillation due to few factors. One is that the distillation column is usually packed with copper, stool wire or glass beads. This increases the surface area, which enables efficient and continuous vaporization and condensation by exchanging heat between the vapor and the condensate. | ||
{{clear}} | {{clear}} | ||
| − | ===p. Filtration === | + | ===p. Filtration === <!--T:38--> |
| + | <!--T:39--> | ||
Filtration is a technique in which a solid precipitate (or solid waste) is separated from a liquid. The mixture is placed on filter paper, which allows the liquid to pass through, leaving the solid behind. Typically the liquid is first brought to a temperature such that there is little solid dissolved in the liquid, so that separation is most effective. This filtration can be done using only gravity or suction can be used. | Filtration is a technique in which a solid precipitate (or solid waste) is separated from a liquid. The mixture is placed on filter paper, which allows the liquid to pass through, leaving the solid behind. Typically the liquid is first brought to a temperature such that there is little solid dissolved in the liquid, so that separation is most effective. This filtration can be done using only gravity or suction can be used. | ||
| − | ==2. What gases extinguish life, and how? Explain the principle of one kind of chemical fire extinguisher.== | + | ==2. What gases extinguish life, and how? Explain the principle of one kind of chemical fire extinguisher.== <!--T:40--> |
| + | <!--T:41--> | ||
In the extreme, almost any gas can kill a person. Even pure oxygen can kill because the body cannot deal effectively with pure oxygen. The gases can kill by: | In the extreme, almost any gas can kill a person. Even pure oxygen can kill because the body cannot deal effectively with pure oxygen. The gases can kill by: | ||
| + | <!--T:42--> | ||
:1) Suffocation- The gas displaces oxygen and the body now starved of oxygen dies. Some of the most dangerous suffocants are oxidation compounds such as <math>CO_2</math> or CO | :1) Suffocation- The gas displaces oxygen and the body now starved of oxygen dies. Some of the most dangerous suffocants are oxidation compounds such as <math>CO_2</math> or CO | ||
| + | <!--T:43--> | ||
:2) Poison- There are gases such as Cyanide gas HCN which are highly poisonous. Cyanide binds to the iron atoms in the enzyme known as cytochrome c oxidase and thus blocks the production of ATP. ATP is the universal energy currency of all living organisms. | :2) Poison- There are gases such as Cyanide gas HCN which are highly poisonous. Cyanide binds to the iron atoms in the enzyme known as cytochrome c oxidase and thus blocks the production of ATP. ATP is the universal energy currency of all living organisms. | ||
| + | <!--T:44--> | ||
:3) Explosion- Many gases are quite flammable and can explode quite destructively. Most of the light Hydrocarbons in mines can be set off with only a small spark. | :3) Explosion- Many gases are quite flammable and can explode quite destructively. Most of the light Hydrocarbons in mines can be set off with only a small spark. | ||
| + | <!--T:45--> | ||
Fire, like life, requires oxygen, so a common method used in fire extinguishers is to use a gas or liquid that will keep the oxygen from getting to the fuel. | Fire, like life, requires oxygen, so a common method used in fire extinguishers is to use a gas or liquid that will keep the oxygen from getting to the fuel. | ||
| − | ==3. Name two common sources of carbon monoxide. Why is it dangerous? == | + | ==3. Name two common sources of carbon monoxide. Why is it dangerous? == <!--T:46--> |
| + | <!--T:47--> | ||
Automobile exhaust before the catalytic converter contains large amounts of CO (Carbon Monoxide). If the exhaust system is damaged before this point, then there is a danger of breathing the suffocating gas. The catalytic converter converts most of the CO to <math>CO_2</math> (Carbon Dioxide) There may still be enough CO in automobile exhaust to be fatal, but since most is now <math>CO_2</math> which has an odor or at least sensation, there is less chance of accidental asphyxiation.( <math>CO_2</math> can be fatal as well, but is quite caustic, creating a burning sensation in the nose and lungs. If you have ever breathed the bubbles from a Soda Pop can, you have smelled <math>CO_2</math>.) | Automobile exhaust before the catalytic converter contains large amounts of CO (Carbon Monoxide). If the exhaust system is damaged before this point, then there is a danger of breathing the suffocating gas. The catalytic converter converts most of the CO to <math>CO_2</math> (Carbon Dioxide) There may still be enough CO in automobile exhaust to be fatal, but since most is now <math>CO_2</math> which has an odor or at least sensation, there is less chance of accidental asphyxiation.( <math>CO_2</math> can be fatal as well, but is quite caustic, creating a burning sensation in the nose and lungs. If you have ever breathed the bubbles from a Soda Pop can, you have smelled <math>CO_2</math>.) | ||
| + | <!--T:48--> | ||
Another common source of CO is natural gas or propane stoves, heaters, hot water heaters, or clothes dryers. If these appliances become damaged they can be quite dangerous. It is a good idea to have a CO detector near an old furnace or water heater to provide an early warning. | Another common source of CO is natural gas or propane stoves, heaters, hot water heaters, or clothes dryers. If these appliances become damaged they can be quite dangerous. It is a good idea to have a CO detector near an old furnace or water heater to provide an early warning. | ||
| + | <!--T:49--> | ||
Oxygen is carried to the cells from the lungs by red blood cells and CO<sub>2</sub> is carried back to the lungs to be breathed out. Red blood cells have a 3-D space that fits oxygen and CO<sub>2</sub> but allows them to be ejected when they reach their destination. CO can also fit the space but won't let go, so a red blood cell that has CO loaded is no longer available to do its job. If enough red blood cells are affected, the body cells cannot get the oxygen they need and waste CO<sub>2</sub> builds up, leading to death. | Oxygen is carried to the cells from the lungs by red blood cells and CO<sub>2</sub> is carried back to the lungs to be breathed out. Red blood cells have a 3-D space that fits oxygen and CO<sub>2</sub> but allows them to be ejected when they reach their destination. CO can also fit the space but won't let go, so a red blood cell that has CO loaded is no longer available to do its job. If enough red blood cells are affected, the body cells cannot get the oxygen they need and waste CO<sub>2</sub> builds up, leading to death. | ||
| − | ==4. What are the states of matter? == | + | ==4. What are the states of matter? == <!--T:50--> |
;[[W:Solids|Solid]] | ;[[W:Solids|Solid]] | ||
:The atoms are in a fairly rigid structure that at the macroscopic level feels hard or solid. The atoms may be arranged in a very rigid crystaline structure, but there is still vibration within the structure. | :The atoms are in a fairly rigid structure that at the macroscopic level feels hard or solid. The atoms may be arranged in a very rigid crystaline structure, but there is still vibration within the structure. | ||
| Line 131: | Line 162: | ||
:The molecules of a plasma are ionized, which causes them to repel each other, so a plasma can appear to flow almost like a liquid, and it glows as some of the molecules change ionization states. | :The molecules of a plasma are ionized, which causes them to repel each other, so a plasma can appear to flow almost like a liquid, and it glows as some of the molecules change ionization states. | ||
| + | <!--T:51--> | ||
The states of matter are largely defined based on the level of interaction between the molecules and atoms that form the matter. This interaction is temperature (and pressure) dependent, and there are well defined freezing (liquid to solid) points, melting(solid to liquid) points, boiling (liquid to gas) points, condensation( gas to liquid) points, for each element or molecule at a particular air pressure(i.e. normal sea level pressure). | The states of matter are largely defined based on the level of interaction between the molecules and atoms that form the matter. This interaction is temperature (and pressure) dependent, and there are well defined freezing (liquid to solid) points, melting(solid to liquid) points, boiling (liquid to gas) points, condensation( gas to liquid) points, for each element or molecule at a particular air pressure(i.e. normal sea level pressure). | ||
| + | <!--T:52--> | ||
''This is why water boils at a lower temperature on a mountain which has less air pressure than at sea level. This can effect the efficiency of sterilizing water by boiling and a longer time is needed.'' | ''This is why water boils at a lower temperature on a mountain which has less air pressure than at sea level. This can effect the efficiency of sterilizing water by boiling and a longer time is needed.'' | ||
| + | <!--T:53--> | ||
''CO<sub>2</sub> is a unusual gas as in its solid form it doesn't melt but goes straight to a gas (sublimate). This is why frozen CO<sub>2</sub> is known as Dry Ice as it does not go through a liquid form so is not 'wet'.'' | ''CO<sub>2</sub> is a unusual gas as in its solid form it doesn't melt but goes straight to a gas (sublimate). This is why frozen CO<sub>2</sub> is known as Dry Ice as it does not go through a liquid form so is not 'wet'.'' | ||
| − | ==5. Do five of the following, and explain the chemical action that takes place: == | + | ==5. Do five of the following, and explain the chemical action that takes place: == <!--T:54--> |
''' | ''' | ||
To Do: Explain the chemical action that takes place for each task.''' | To Do: Explain the chemical action that takes place for each task.''' | ||
| − | ===a. Try to light a sugar cube, first without and then with some ash applied to the cube, thus showing the action of a catalyst. === | + | ===a. Try to light a sugar cube, first without and then with some ash applied to the cube, thus showing the action of a catalyst. === <!--T:55--> |
| + | <!--T:56--> | ||
;Materials: | ;Materials: | ||
*Sugar Cubes | *Sugar Cubes | ||
| Line 158: | Line 193: | ||
*Try to light each sugar cube. It should be easier to light the ash-coated sugar cube. | *Try to light each sugar cube. It should be easier to light the ash-coated sugar cube. | ||
| − | ===b. Place an ice cube in a glass of water, place a four-inch (10.2 cm) string on top of the glass and ice, then solve the problem of taking the ice cube out of the water without touching it. === | + | ===b. Place an ice cube in a glass of water, place a four-inch (10.2 cm) string on top of the glass and ice, then solve the problem of taking the ice cube out of the water without touching it. === <!--T:57--> |
| + | <!--T:58--> | ||
;Materials | ;Materials | ||
*Ice | *Ice | ||
| Line 172: | Line 208: | ||
*Wait a while then pull up the string | *Wait a while then pull up the string | ||
| − | ===c. With the use of water, turpentine, and soap, transfer a newspaper picture to a blank sheet of paper. === | + | ===c. With the use of water, turpentine, and soap, transfer a newspaper picture to a blank sheet of paper. === <!--T:59--> |
| + | <!--T:60--> | ||
;Materials: | ;Materials: | ||
* 30 ml soap powder (example: Ivory Snow) | * 30 ml soap powder (example: Ivory Snow) | ||
| Line 183: | Line 220: | ||
* Newspaper or old magazine | * Newspaper or old magazine | ||
| + | <!--T:61--> | ||
;Method: | ;Method: | ||
| + | <!--T:62--> | ||
* Dissolve soap powder in hot water and then add turpentine. | * Dissolve soap powder in hot water and then add turpentine. | ||
* To use, dip a brush into the ink and brush over the picture to be transferred, wait about ten seconds then place a piece of paper over the picture and rub the back of it with a spoon. The picture will be transferred to the paper. | * To use, dip a brush into the ink and brush over the picture to be transferred, wait about ten seconds then place a piece of paper over the picture and rub the back of it with a spoon. The picture will be transferred to the paper. | ||
| + | <!--T:63--> | ||
The ink will solidify in its container after a little while. To reverse this simply set the bottle in a pan of warm water until melted and then shake. | The ink will solidify in its container after a little while. To reverse this simply set the bottle in a pan of warm water until melted and then shake. | ||
| − | ===d. With the use of a candle and a piece of cardboard, demonstrate visually the three parts of a candle flame. === | + | ===d. With the use of a candle and a piece of cardboard, demonstrate visually the three parts of a candle flame. === <!--T:64--> |
[[Image:Kaarsvlam-kl.jpg|thumb|left|200px]] | [[Image:Kaarsvlam-kl.jpg|thumb|left|200px]] | ||
| + | <!--T:65--> | ||
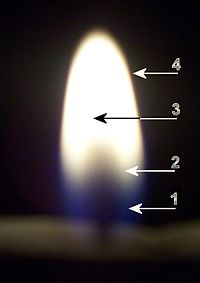
;1) Blue zone : The blue area is the base of the flame. In this area, '''[[W:Pyrolysis|pyrolysis]]''' takes place (where the candle wax ''changes state'' into the combustible gas). Also, part of ''combustion'' takes place here. The temperature in this area is about 1200-1400ºC. | ;1) Blue zone : The blue area is the base of the flame. In this area, '''[[W:Pyrolysis|pyrolysis]]''' takes place (where the candle wax ''changes state'' into the combustible gas). Also, part of ''combustion'' takes place here. The temperature in this area is about 1200-1400ºC. | ||
| + | <!--T:66--> | ||
;2) Dark zone : The dark area in the middle of the flame just above the tip. This dark core of the flame is around 800-1000ºC. Within these bluer regions, hydrogen is being separated from the fuel and burned to form water vapor. | ;2) Dark zone : The dark area in the middle of the flame just above the tip. This dark core of the flame is around 800-1000ºC. Within these bluer regions, hydrogen is being separated from the fuel and burned to form water vapor. | ||
| + | <!--T:67--> | ||
;3) Luminous zone : The yellow luminous area is above the dark area. This brighter, yellower part of the flame is the remaining carbon being oxidized to form carbon dioxide. The incandescent soot particles causes the orange and yellow glow. This area is approximately 1200ºC. | ;3) Luminous zone : The yellow luminous area is above the dark area. This brighter, yellower part of the flame is the remaining carbon being oxidized to form carbon dioxide. The incandescent soot particles causes the orange and yellow glow. This area is approximately 1200ºC. | ||
| + | <!--T:68--> | ||
;4) Flame Mantle : this is on the outer rim of the flame, and is colorless, or a very faint blue, and is the hottest part of the candle flame. About 1400ºC. | ;4) Flame Mantle : this is on the outer rim of the flame, and is colorless, or a very faint blue, and is the hottest part of the candle flame. About 1400ºC. | ||
| − | ===e. With a bowl of water, wooden match sticks, a lump of sugar, and small amount soap, demonstrate the action of sugar and soap on the floating match sticks. === | + | ===e. With a bowl of water, wooden match sticks, a lump of sugar, and small amount soap, demonstrate the action of sugar and soap on the floating match sticks. === <!--T:69--> |
| + | <!--T:70--> | ||
;Materials: | ;Materials: | ||
* Sugar cube | * Sugar cube | ||
| Line 210: | Line 255: | ||
* Water | * Water | ||
| + | <!--T:71--> | ||
;Method: | ;Method: | ||
*Fill both bowls water. | *Fill both bowls water. | ||
| Line 216: | Line 262: | ||
*Place one drop of dish detergent in the other bowl. The toothpicks should be repelled from it. | *Place one drop of dish detergent in the other bowl. The toothpicks should be repelled from it. | ||
| + | <!--T:72--> | ||
Sugar absorbs water, and as it does, it creates a small current that draws the toothpicks toward it. The Soap, on the other hand, breaks the surface tension of the water and immediately spreads out over the surface. As it moves across the surface, it too creates a current, carrying the toothpicks along as it goes. | Sugar absorbs water, and as it does, it creates a small current that draws the toothpicks toward it. The Soap, on the other hand, breaks the surface tension of the water and immediately spreads out over the surface. As it moves across the surface, it too creates a current, carrying the toothpicks along as it goes. | ||
| − | ===f. Place a fresh egg in fresh water and then salt water, noting the difference.=== | + | ===f. Place a fresh egg in fresh water and then salt water, noting the difference.=== <!--T:73--> |
;Materials | ;Materials | ||
* Fresh (uncooked) egg | * Fresh (uncooked) egg | ||
| Line 225: | Line 272: | ||
* 2 Bowls | * 2 Bowls | ||
| + | <!--T:74--> | ||
;Methods | ;Methods | ||
*Add the salt and {{units|a half liter|2 cups}} of water to a bowl and stir until the salt dissolves. | *Add the salt and {{units|a half liter|2 cups}} of water to a bowl and stir until the salt dissolves. | ||
| Line 231: | Line 279: | ||
*Move the egg to the unsalted water. It should sink. | *Move the egg to the unsalted water. It should sink. | ||
| + | <!--T:75--> | ||
Salt water is more dense than fresh water, meaning that a volume of salt water will weigh more than an equal volume of fresh water. The density of an egg is between the density of salt water and fresh water. An item will float if it is less dense than the liquid in which it is placed. Since the egg is more dense than fresh water, it sinks. But because the egg is less dense than the salt water, it floats. NOTE: If nothing happens, then get two eggs and place one in the fresh water and the other in the salt water. The fresh water egg should expand and the salt water egg should shrink due to osmosis. | Salt water is more dense than fresh water, meaning that a volume of salt water will weigh more than an equal volume of fresh water. The density of an egg is between the density of salt water and fresh water. An item will float if it is less dense than the liquid in which it is placed. Since the egg is more dense than fresh water, it sinks. But because the egg is less dense than the salt water, it floats. NOTE: If nothing happens, then get two eggs and place one in the fresh water and the other in the salt water. The fresh water egg should expand and the salt water egg should shrink due to osmosis. | ||
| − | ===g. Demonstrate that rust uses up oxygen with the use of steel wool, a pencil, a rubber band, a water glass, and a dish of water. === | + | ===g. Demonstrate that rust uses up oxygen with the use of steel wool, a pencil, a rubber band, a water glass, and a dish of water. === <!--T:76--> |
;Materials | ;Materials | ||
*Steel wool (plain, without soap) | *Steel wool (plain, without soap) | ||
| Line 242: | Line 291: | ||
*Water | *Water | ||
| + | <!--T:77--> | ||
;Method | ;Method | ||
Use the rubber band to attach the steel wool to the eraser end of the pencil. The pencil should be short enough that it can fit inside the glass without sticking out the top. Fill the glass halfway with water, and place the pencil in the glass with the wad of steel wool at the bottom. Place a bowl upside-down over the top of the glass, and carefully turn the glass and the bowl over so that the glass is upside-down and the bowl is right-side-up. Add some water to the bowl and mark the water level on the side of the glass. Place the apparatus some place where it will not be disturbed. After a few days, check the steel wool - it should be rusty. | Use the rubber band to attach the steel wool to the eraser end of the pencil. The pencil should be short enough that it can fit inside the glass without sticking out the top. Fill the glass halfway with water, and place the pencil in the glass with the wad of steel wool at the bottom. Place a bowl upside-down over the top of the glass, and carefully turn the glass and the bowl over so that the glass is upside-down and the bowl is right-side-up. Add some water to the bowl and mark the water level on the side of the glass. Place the apparatus some place where it will not be disturbed. After a few days, check the steel wool - it should be rusty. | ||
| + | <!--T:78--> | ||
As the steel wool rusts, it uses up oxygen trapped in the glass. This reduces the volume of air inside the glass, and the atmospheric pressure outside the glass will compensate by pushing down on the water inside the bowl. This will in turn push the water in the glass higher. Verify by comparing the current water level to the level marked at the beginning of the experiment. | As the steel wool rusts, it uses up oxygen trapped in the glass. This reduces the volume of air inside the glass, and the atmospheric pressure outside the glass will compensate by pushing down on the water inside the bowl. This will in turn push the water in the glass higher. Verify by comparing the current water level to the level marked at the beginning of the experiment. | ||
| − | ===h. Demonstrate the colors produced when the following are burned: salt, copper sulfate, and boric acid. === | + | ===h. Demonstrate the colors produced when the following are burned: salt, copper sulfate, and boric acid. === <!--T:79--> |
* '''Salt''' : Common table salt is ''Sodium Chloride'' [http://jchemed.chem.wisc.edu/jcesoft/cca/cca2/STHTM/FLAME/9.HTM Orange-Yellow] | * '''Salt''' : Common table salt is ''Sodium Chloride'' [http://jchemed.chem.wisc.edu/jcesoft/cca/cca2/STHTM/FLAME/9.HTM Orange-Yellow] | ||
* '''Copper Sulfate''' : Blue-Green | * '''Copper Sulfate''' : Blue-Green | ||
| Line 258: | Line 309: | ||
| + | <!--T:80--> | ||
'''More Colors/Flames''' (because they are colorful) | '''More Colors/Flames''' (because they are colorful) | ||
<gallery> | <gallery> | ||
| Line 273: | Line 325: | ||
</gallery> | </gallery> | ||
| − | ===i. Make an invisible ink. === | + | ===i. Make an invisible ink. === <!--T:81--> |
| + | <!--T:82--> | ||
;Materials | ;Materials | ||
*Water | *Water | ||
| Line 283: | Line 336: | ||
*Paper | *Paper | ||
| + | <!--T:83--> | ||
;Methods | ;Methods | ||
You can mix vinegar, lemon juice, sugar or onion juice with water to create an invisible ink. To make the ink visible, carefully hold it over a heat source. The "ink" will turn colors and become visible. Try mixing a small amount of ink using each type of ingredients to see which works better. | You can mix vinegar, lemon juice, sugar or onion juice with water to create an invisible ink. To make the ink visible, carefully hold it over a heat source. The "ink" will turn colors and become visible. Try mixing a small amount of ink using each type of ingredients to see which works better. | ||
| − | ===j. Show that washing soda or sodium carbonate contains water.=== | + | ===j. Show that washing soda or sodium carbonate contains water.=== <!--T:84--> |
;Materials | ;Materials | ||
*Test tube/tongs | *Test tube/tongs | ||
| Line 292: | Line 346: | ||
*Candle or stove flame | *Candle or stove flame | ||
| + | <!--T:85--> | ||
;Methods | ;Methods | ||
Put a small amount of the washing soda into the test tube. Hold the test tube with the tongs over a flame. You will see a tornado-like effect inside the test tube as the water makes its "escape". Very fun for everyone to watch! | Put a small amount of the washing soda into the test tube. Hold the test tube with the tongs over a flame. You will see a tornado-like effect inside the test tube as the water makes its "escape". Very fun for everyone to watch! | ||
| − | ==Historical Notes== | + | ==Historical Notes== <!--T:86--> |
| + | <!--T:87--> | ||
This Honor was previously classed as a Nature Honor with a white background. | This Honor was previously classed as a Nature Honor with a white background. | ||
| − | ==References== | + | ==References== <!--T:88--> |
<noinclude></translate></noinclude> | <noinclude></translate></noinclude> | ||
[[Category:Adventist Youth Honors Answer Book/Completed Honors|{{SUBPAGENAME}}]] | [[Category:Adventist Youth Honors Answer Book/Completed Honors|{{SUBPAGENAME}}]] | ||
Revision as of 05:38, 4 December 2014
1. Define the following terms:
a. Elements
A class of substances that cannot be separated into simpler substances by chemical means.
b. Compounds
A uniform substance composed of two or more elements.
c. Chemical symbols
Every element is represented using an abbreviation of one or two characters that represent the name of the element. The first character is always capitalized, and the second character if it exists is always lower case.
- H represents hydrogen
- He represents helium
- Li represents lithium
- C represents Carbon
Some of the symbols do not represent the modern name, but an original name.
- Ag represents argentum (Latin) or silver
- Au represents aurum (Latin) or gold
- W represents wolfram (German) or tungsten
There are 94 elements that are found naturally on earth, and there are another 17 that have been created in atomic accelerators, and have very short half lives. A further 7 elements have been reported but they are still in the confirming/naming stage. All of these elements are organized using a "Periodic Table of Elements" which was created by Dimitri Mendeleev.
d. Solutions
A mixture of two or more substances that do not react chemically. If you dissolve sugar, salt, or another substance in water, you are creating a solution.
e. Atoms
Atoms are basic building blocks of matter. Atoms range in size from .5Å to 24Å. ( Å is the symbol for a unit of measure known as an angstrom. A blond hair is between 170,000 and 500,000Å in diameter and a black hair is between 560,000 and 1,810,000Å in diameter)
Atoms are mostly empty space, but because the electrons are moving so rapidly, the matter we touch can feel very solid. Hydrogen is the simplest atom, it is made of one proton and one electron. A classical model of an atom is to think about the electron orbiting the central proton. If we created a model of this atom with the proton the size of a basket ball, then the electron would be the size of a grain of salt and will be orbiting at a distance of 40,000 feet.
We cannot directly see atoms with a regular microscope. Light has a wavelength of between 4,000Å and 7,000Å which is about 1000 times the diameter of an atom. Because the wavelength of light is so long it has almost no interaction with the atom. The center of an Atom can be "viewed" and the positions of the electron cloud around an atom can be determined by using an X-ray, (neutron, or electron) beam (which have much smaller wavelengths than light) in such techniques as X-ray crystallography, Neutron crystallography, or Electron crystallography. Or a scanning tunneling microscope can be used.
f. Molecules
Two or more atoms that have bonded to each other.
g. Periodic Table
The periodic table of the chemical elements is a tabular method of displaying the chemical elements. The table illustrates recurring ("periodic") trends in the properties of the elements.
| Group → | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||
| ↓ Period | ||||||||||||||||||||
| 1 | 1 H |
2 He | ||||||||||||||||||
| 2 | 3 Li |
4 Be |
5 B |
6 C |
7 N |
8 O |
9 F |
10 Ne | ||||||||||||
| 3 | 11 Na |
12 Mg |
13 Al |
14 Si |
15 P |
16 S |
17 Cl |
18 Ar | ||||||||||||
| 4 | 19 K |
20 Ca |
21 Sc |
22 Ti |
23 V |
24 Cr |
25 Mn |
26 Fe |
27 Co |
28 Ni |
29 Cu |
30 Zn |
31 Ga |
32 Ge |
33 As |
34 Se |
35 Br |
36 Kr | ||
| 5 | 37 Rb |
38 Sr |
39 Y |
40 Zr |
41 Nb |
42 Mo |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 In |
50 Sn |
51 Sb |
52 Te |
53 I |
54 Xe | ||
| 6 | 55 Cs |
56 Ba |
57 La |
58 Ce |
* |
72 Hf |
73 Ta |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 Hg |
81 Tl |
82 Pb |
83 Bi |
84 Po |
85 At |
86 Rn |
| 7 | 87 Fr |
88 Ra |
89 Ac |
90 Th |
** |
104 Rf |
105 Db |
106 Sg |
107 Bh |
108 Hs |
109 Mt |
110 Ds |
111 Rg |
112 Cn |
113 Uut |
114 Uuq |
115 Uup |
116 Uuh |
117 Uus |
118 Uuo |
| * Lanthanides | 59 Pr |
60 Nd |
61 Pm |
62 Sm |
63 Eu |
64 Gd |
65 Tb |
66 Dy |
67 Ho |
68 Er |
69 Tm |
70 Yb |
71 Lu | |||||||
| ** Actinides | 91 Pa |
92 U |
93 Np |
94 Pu |
95 Am |
96 Cm |
97 Bk |
98 Cf |
99 Es |
100 Fm |
101 Md |
102 No |
103 Lr | |||||||
| Alkali metals2 | Alkaline earth metals2 | Lanthanides12 | Actinides12 | Transition metals2 |
| Poor metals | Metalloids | Nonmetals | Halogens3 | Noble gases3 |
1Actinides and lanthanides are collectively known as "Rare Earth Metals." 2Alkali metals, alkaline Earth metals, transition metals, actinides, and lanthanides are all collectively known as "Metals." 3Halogens and noble gases are also non-metals.
State at standard temperature and pressure
- those with atomic number in blue are not known at STP
- those with atomic number in red are gases at standard temperature and pressure (STP)
- those with atomic number in green are liquids at STP
- those with atomic number in black are solid at STP
Natural occurrence
- those with solid borders have isotopes that are older than the Earth (Primordial elements)
- those with dashed borders naturally arise from decay of other chemical elements and have no isotopes older than the earth
- those with dotted borders are made artificially (Synthetic elements)
Unknown properties
- those with a cyan background have unknown chemical properties.
h. Combustion
We often use the word combustion to describe the chemical process of a fuel combining rapidly with a oxidizer (usually oxygen)i.e. burning. This process usually is associated with flames, light, heat, and smoke.
i. Acid
The word acid is from the Latin word acidus which means sour. In the early days of chemistry, it was a common practice to taste or smell things and record the sensation. Many things that are acidic are sour. Lemon juice is sour because of the citric acid in it.
Because many substances can be toxic, this probably killed a number of chemists including the famous scientist, Humphry Davy, the man who came up with the theory that explained the nature of an acid.
Davy's hypothesis was that acids were substances that contained replaceable hydrogen. This hydrogen could be replaced by metals and this reaction would leave behind a salt.
- 2HCl + Zn → ZnCl2 + H2
- Fe + H2SO4 → H2 + FeSO4
j. Salt
A salt is a term for the solid form of an ionic compound, such as Sodium Chloride (Na+Cl-) or potassium hydroxide (K+(OH)-).
k. Proton
A positively charged particle that is a part of the nucleus of an atom. Protons have a mass of [math]\displaystyle{ 1.67 \times 10^{-27} }[/math] kg. One cubic centimeter of water contains [math]\displaystyle{ 6.02 \times 10^{24} }[/math]protons that form the nucleus of the atoms of hydrogen and oxygen. Hydrogen has one proton at its center and oxygen has 8 protons.
l. Neutron
A neutrally charged particle that is a part of the nucleus of an atom. Neutron's have a mass of [math]\displaystyle{ 1.67 \times 10^{-27} }[/math] kg. When we look at water we find that it is made up of Hydrogen and Oxygen. Hydrogen has no neutrons, but each atom of oxygen has 8 neutrons.
Neutrons are held together by what is called the "Weak Force". Free neutrons decay with a half life of about 10.3 minutes into a proton, electron and an electron nutrino. In the neucleus of an atom, the neutrons can be quite stable because of another force known as the "Strong Force"
m. Electron
A negatively charged particle with a mass of [math]\displaystyle{ 9.11 \times 10^{-31} }[/math] kg. Electrons form a cloud about the positively charged nucleus of an atom.
n. Distillation
Distillation, which is heating the liquid to boiling and collecting the condensed vapors, is a useful technique to separate components based on different boiling points. Distillation separates two volatile liquids where the more volatile component is more pure. The farther apart the boiling temperatures, the more likely that the distillation will be successful. The lower the boiling point, the more volatile the compound is and therefore the faster it will vaporize. Success also depends on the interactions between particles, since two dissimilar liquids that are dissolved in each other are likely to form azeotropes. If this occurs, the liquid and gas phase will both have the same composition and therefore boiling will not cause further separation.
o. Fractional Distillation
Fractional distillation is a technique in which several distillations occur in the same column, mediated by some sort of porous medium. It allows the separation of substances with closer boiling points than simple distillation, and also makes the distillation of the same solutions more efficient. Fractional distillation yields better separation in comparison to simple distillation due to few factors. One is that the distillation column is usually packed with copper, stool wire or glass beads. This increases the surface area, which enables efficient and continuous vaporization and condensation by exchanging heat between the vapor and the condensate.
p. Filtration
Filtration is a technique in which a solid precipitate (or solid waste) is separated from a liquid. The mixture is placed on filter paper, which allows the liquid to pass through, leaving the solid behind. Typically the liquid is first brought to a temperature such that there is little solid dissolved in the liquid, so that separation is most effective. This filtration can be done using only gravity or suction can be used.
2. What gases extinguish life, and how? Explain the principle of one kind of chemical fire extinguisher.
In the extreme, almost any gas can kill a person. Even pure oxygen can kill because the body cannot deal effectively with pure oxygen. The gases can kill by:
- 1) Suffocation- The gas displaces oxygen and the body now starved of oxygen dies. Some of the most dangerous suffocants are oxidation compounds such as [math]\displaystyle{ CO_2 }[/math] or CO
- 2) Poison- There are gases such as Cyanide gas HCN which are highly poisonous. Cyanide binds to the iron atoms in the enzyme known as cytochrome c oxidase and thus blocks the production of ATP. ATP is the universal energy currency of all living organisms.
- 3) Explosion- Many gases are quite flammable and can explode quite destructively. Most of the light Hydrocarbons in mines can be set off with only a small spark.
Fire, like life, requires oxygen, so a common method used in fire extinguishers is to use a gas or liquid that will keep the oxygen from getting to the fuel.
3. Name two common sources of carbon monoxide. Why is it dangerous?
Automobile exhaust before the catalytic converter contains large amounts of CO (Carbon Monoxide). If the exhaust system is damaged before this point, then there is a danger of breathing the suffocating gas. The catalytic converter converts most of the CO to [math]\displaystyle{ CO_2 }[/math] (Carbon Dioxide) There may still be enough CO in automobile exhaust to be fatal, but since most is now [math]\displaystyle{ CO_2 }[/math] which has an odor or at least sensation, there is less chance of accidental asphyxiation.( [math]\displaystyle{ CO_2 }[/math] can be fatal as well, but is quite caustic, creating a burning sensation in the nose and lungs. If you have ever breathed the bubbles from a Soda Pop can, you have smelled [math]\displaystyle{ CO_2 }[/math].)
Another common source of CO is natural gas or propane stoves, heaters, hot water heaters, or clothes dryers. If these appliances become damaged they can be quite dangerous. It is a good idea to have a CO detector near an old furnace or water heater to provide an early warning.
Oxygen is carried to the cells from the lungs by red blood cells and CO2 is carried back to the lungs to be breathed out. Red blood cells have a 3-D space that fits oxygen and CO2 but allows them to be ejected when they reach their destination. CO can also fit the space but won't let go, so a red blood cell that has CO loaded is no longer available to do its job. If enough red blood cells are affected, the body cells cannot get the oxygen they need and waste CO2 builds up, leading to death.
4. What are the states of matter?
- Solid
- The atoms are in a fairly rigid structure that at the macroscopic level feels hard or solid. The atoms may be arranged in a very rigid crystaline structure, but there is still vibration within the structure.
- Liquid
- The atoms are free to move around, but do not separate like a gas. Liquids can flow and in general do not feel solid or rigid. Liquids are not compressible. When helium is cooled close to absolute zero, it behaves oddly, turning into a superfluid. It is dangerous to handle because of its temperature.
- Gas
- The molecules of a gas are completely free to move around and at standard room temperature they move tens of meters before hitting another molecule. A gas is airy. You will not necessarily know that it is there unless you move through it and feel the wind.
- Plasma
- The molecules of a plasma are ionized, which causes them to repel each other, so a plasma can appear to flow almost like a liquid, and it glows as some of the molecules change ionization states.
The states of matter are largely defined based on the level of interaction between the molecules and atoms that form the matter. This interaction is temperature (and pressure) dependent, and there are well defined freezing (liquid to solid) points, melting(solid to liquid) points, boiling (liquid to gas) points, condensation( gas to liquid) points, for each element or molecule at a particular air pressure(i.e. normal sea level pressure).
This is why water boils at a lower temperature on a mountain which has less air pressure than at sea level. This can effect the efficiency of sterilizing water by boiling and a longer time is needed.
CO2 is a unusual gas as in its solid form it doesn't melt but goes straight to a gas (sublimate). This is why frozen CO2 is known as Dry Ice as it does not go through a liquid form so is not 'wet'.
5. Do five of the following, and explain the chemical action that takes place:
To Do: Explain the chemical action that takes place for each task.
a. Try to light a sugar cube, first without and then with some ash applied to the cube, thus showing the action of a catalyst.
- Materials
- Sugar Cubes
- Ash from fireplace or campfire
- Matches or lighter
- Aluminum foil
- Piece of wood or trivet to act as insulator
- Method
- Lay a piece of aluminum over the trivet or a piece of wood.
- Place the sugar cube near the center of the foil.
- Take another sugar cube and coat it with ash.
- Place the ash-coated sugar cube next to the first sugar cube, but do not let them touch.
- Try to light each sugar cube. It should be easier to light the ash-coated sugar cube.
b. Place an ice cube in a glass of water, place a four-inch (10.2 cm) string on top of the glass and ice, then solve the problem of taking the ice cube out of the water without touching it.
- Materials
- Ice
- Salt
- Water
- Glass
- String 10 cm long
- Method
- Put the ice cube in the glass
- Tie a loop on the string and place it on the cube
- Put a pinch of salt on the loop and cube
- Wait a while then pull up the string
c. With the use of water, turpentine, and soap, transfer a newspaper picture to a blank sheet of paper.
- Materials
- 30 ml soap powder (example: Ivory Snow)
- 60 ml hot water
- 15 ml turpentine
- Small bowl
- Measuring cup
- Paint brush
- Newspaper or old magazine
- Method
- Dissolve soap powder in hot water and then add turpentine.
- To use, dip a brush into the ink and brush over the picture to be transferred, wait about ten seconds then place a piece of paper over the picture and rub the back of it with a spoon. The picture will be transferred to the paper.
The ink will solidify in its container after a little while. To reverse this simply set the bottle in a pan of warm water until melted and then shake.
d. With the use of a candle and a piece of cardboard, demonstrate visually the three parts of a candle flame.
- 1) Blue zone
- The blue area is the base of the flame. In this area, pyrolysis takes place (where the candle wax changes state into the combustible gas). Also, part of combustion takes place here. The temperature in this area is about 1200-1400ºC.
- 2) Dark zone
- The dark area in the middle of the flame just above the tip. This dark core of the flame is around 800-1000ºC. Within these bluer regions, hydrogen is being separated from the fuel and burned to form water vapor.
- 3) Luminous zone
- The yellow luminous area is above the dark area. This brighter, yellower part of the flame is the remaining carbon being oxidized to form carbon dioxide. The incandescent soot particles causes the orange and yellow glow. This area is approximately 1200ºC.
- 4) Flame Mantle
- this is on the outer rim of the flame, and is colorless, or a very faint blue, and is the hottest part of the candle flame. About 1400ºC.
e. With a bowl of water, wooden match sticks, a lump of sugar, and small amount soap, demonstrate the action of sugar and soap on the floating match sticks.
- Materials
- Sugar cube
- Dish soap
- 2 Small bowls
- 12 Toothpicks (or wooden match sticks)
- Water
- Method
- Fill both bowls water.
- Drop half the toothpicks into each bowl
- Place the sugar cube in one bowl. The toothpicks should be drawn to it.
- Place one drop of dish detergent in the other bowl. The toothpicks should be repelled from it.
Sugar absorbs water, and as it does, it creates a small current that draws the toothpicks toward it. The Soap, on the other hand, breaks the surface tension of the water and immediately spreads out over the surface. As it moves across the surface, it too creates a current, carrying the toothpicks along as it goes.
f. Place a fresh egg in fresh water and then salt water, noting the difference.
- Materials
- Methods
- Add the salt and a half liter
 of water to a bowl and stir until the salt dissolves.
of water to a bowl and stir until the salt dissolves. - Add the other half liter
 of water to the other bowl - do not add any salt.
of water to the other bowl - do not add any salt. - Place the egg in the salted water. It should float
- Move the egg to the unsalted water. It should sink.
Salt water is more dense than fresh water, meaning that a volume of salt water will weigh more than an equal volume of fresh water. The density of an egg is between the density of salt water and fresh water. An item will float if it is less dense than the liquid in which it is placed. Since the egg is more dense than fresh water, it sinks. But because the egg is less dense than the salt water, it floats. NOTE: If nothing happens, then get two eggs and place one in the fresh water and the other in the salt water. The fresh water egg should expand and the salt water egg should shrink due to osmosis.
g. Demonstrate that rust uses up oxygen with the use of steel wool, a pencil, a rubber band, a water glass, and a dish of water.
- Materials
- Steel wool (plain, without soap)
- Pencil
- Rubber band
- Drinking glass (clear glass)
- Bowl
- Water
- Method
Use the rubber band to attach the steel wool to the eraser end of the pencil. The pencil should be short enough that it can fit inside the glass without sticking out the top. Fill the glass halfway with water, and place the pencil in the glass with the wad of steel wool at the bottom. Place a bowl upside-down over the top of the glass, and carefully turn the glass and the bowl over so that the glass is upside-down and the bowl is right-side-up. Add some water to the bowl and mark the water level on the side of the glass. Place the apparatus some place where it will not be disturbed. After a few days, check the steel wool - it should be rusty.
As the steel wool rusts, it uses up oxygen trapped in the glass. This reduces the volume of air inside the glass, and the atmospheric pressure outside the glass will compensate by pushing down on the water inside the bowl. This will in turn push the water in the glass higher. Verify by comparing the current water level to the level marked at the beginning of the experiment.
h. Demonstrate the colors produced when the following are burned: salt, copper sulfate, and boric acid.
- Salt : Common table salt is Sodium Chloride Orange-Yellow
- Copper Sulfate : Blue-Green
- Boric Acid : Bright Green
More Colors/Flames (because they are colorful)
i. Make an invisible ink.
- Materials
- Water
- Vinegar, Lemon juice, sugar, onion
- Small cups
- Candle or stove flame
- Tooth picks
- Paper
- Methods
You can mix vinegar, lemon juice, sugar or onion juice with water to create an invisible ink. To make the ink visible, carefully hold it over a heat source. The "ink" will turn colors and become visible. Try mixing a small amount of ink using each type of ingredients to see which works better.
j. Show that washing soda or sodium carbonate contains water.
- Materials
- Test tube/tongs
- washing soda (baking soda does pretty much exactly the same thing, but the requirements say washing soda)
- Candle or stove flame
- Methods
Put a small amount of the washing soda into the test tube. Hold the test tube with the tongs over a flame. You will see a tornado-like effect inside the test tube as the water makes its "escape". Very fun for everyone to watch!
Historical Notes
This Honor was previously classed as a Nature Honor with a white background.