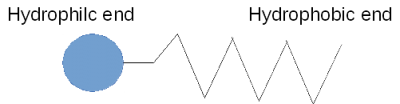Difference between revisions of "AY Honors/Bubbles/Answer Key/en"
(Updating to match new version of source page) |
(Updating to match new version of source page) |
||
| Line 38: | Line 38: | ||
<noinclude></noinclude> | <noinclude></noinclude> | ||
Low humidity equals low life of a bubble. Higher humidity is the key. Even ordinary solutions will perform better in high humidity. Humidity is even more important than temperature, though cold air tends to be less humid than warm air. | Low humidity equals low life of a bubble. Higher humidity is the key. Even ordinary solutions will perform better in high humidity. Humidity is even more important than temperature, though cold air tends to be less humid than warm air. | ||
| − | Good Conditions: | + | Good Conditions: 60% and higher humidity. |
<noinclude></noinclude> | <noinclude></noinclude> | ||
| Line 78: | Line 78: | ||
<!-- 4. List safety rules about bubble blowing. --> | <!-- 4. List safety rules about bubble blowing. --> | ||
*Soap solution will sting your eyes- avoid getting it in your eyes. | *Soap solution will sting your eyes- avoid getting it in your eyes. | ||
| − | *Soap Solution is very Slippery- | + | *Soap Solution is very Slippery- areas might still be slippery long after you are gone, so clean up any spills |
*Never make bubbles near moving traffic. | *Never make bubbles near moving traffic. | ||
*Soap solution can damage grass- dilute with water when finished. | *Soap solution can damage grass- dilute with water when finished. | ||
| Line 100: | Line 100: | ||
{{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=5b}} <!--T:13--> | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=5b}} <!--T:13--> | ||
<noinclude></noinclude> | <noinclude></noinclude> | ||
| − | ====Experiment #1 | + | ====Experiment #1 Float a Paper Clip==== |
*Gently place a DRY paper clip flat onto a small piece of paper towel or use a bend paper clip in an “L” shape. | *Gently place a DRY paper clip flat onto a small piece of paper towel or use a bend paper clip in an “L” shape. | ||
*Slowly lower the paper into the water. | *Slowly lower the paper into the water. | ||
| Line 123: | Line 123: | ||
Possible Variant: Perform this experiment with identical glasses, but use different types of coins in each glass. Use the results of how many can go in to determine a ratio of the volumes of different coins | Possible Variant: Perform this experiment with identical glasses, but use different types of coins in each glass. Use the results of how many can go in to determine a ratio of the volumes of different coins | ||
| − | ====Experiment #3 | + | ====Experiment #3 Floating Needle==== |
Another nice surface tension trick, this one makes it so that a needle will float on the surface of a glass of water. There are two variants of this trick, both impressive in their own right. | Another nice surface tension trick, this one makes it so that a needle will float on the surface of a glass of water. There are two variants of this trick, both impressive in their own right. | ||
| Line 190: | Line 190: | ||
{{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=7d}} <!--T:34--> | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=7d}} <!--T:34--> | ||
<noinclude></noinclude> | <noinclude></noinclude> | ||
| − | '''Water''' | + | '''Water''' Use good quality water that does not contain high levels of iron or minerals. |
'''Soap''' When it comes to soap, Dawn dish soap seems to work the best for homemade bubble solution. | '''Soap''' When it comes to soap, Dawn dish soap seems to work the best for homemade bubble solution. | ||
| Line 233: | Line 233: | ||
White light is made up of all colors, all wavelengths. If one of these colors is subtracted from white light (by interference, for example) we see the complementary color. | White light is made up of all colors, all wavelengths. If one of these colors is subtracted from white light (by interference, for example) we see the complementary color. | ||
| − | Interference | + | Interference: All waves including light, have a curious property. If two waves combine, the waves meet each other crest to crest, adding up and reinforcing the effect of each other or they can meet crest to trough canceling each other out so that they have no effect. |
[[File:Interference of two waves.svg|250px]] | [[File:Interference of two waves.svg|250px]] | ||
Revision as of 22:51, 27 May 2021
1
1a
1b
1c
1d
1e
1f
2
2a
Low humidity equals low life of a bubble. Higher humidity is the key. Even ordinary solutions will perform better in high humidity. Humidity is even more important than temperature, though cold air tends to be less humid than warm air.
Good Conditions: 60% and higher humidity.
2b
When you increase the temperature of a bubble solution, the molecules in the liquid and the gas inside the bubble are moving more quickly. This can cause the solution to thin faster. Also the film that forms the bubble will evaporate more quickly, causing it to pop.
2c
More wind puts a stain on the bubble and dries out the bubble. The main effect of wind is pretty simple and obvious: it puts a mechanical strain on the bubble and is likely to burst it.
The second effect of wind is in helping evaporation.
2d
Hail is totally destructive of bubbles, as is heavy rain. Gentle rain will make a thick raft of bubbles thin down, by diluting and draining the soap solution from the top layers.
3
A soap molecule has a hydrophobic end and a hydrophilic end. In other words, it is both hydrophilic and hydrophobic, and because of this, it corrals water, separating it from the air as shown here.
4
- Soap solution will sting your eyes- avoid getting it in your eyes.
- Soap Solution is very Slippery- areas might still be slippery long after you are gone, so clean up any spills
- Never make bubbles near moving traffic.
- Soap solution can damage grass- dilute with water when finished.
- Don’t drink the bubble solution
5
5a
Surface Tension is the property of the surface of a liquid that allows it to resist an external force.
When you blow a soap bubble, you are creating a pressurized bubble of air which is contained within a thin, elastic surface of liquid.
In a soap-and-water solution the hydrophobic ends of the soap molecule do not want to be in the liquid at all so they find their way to the surface and push their hydrophobic ends out of the water. This separates the water molecules from each other.
Since the surface tension forces become smaller as the distance between water molecules increases, the intervening soap molecules decrease the surface tension.
5b
Experiment #1 Float a Paper Clip
- Gently place a DRY paper clip flat onto a small piece of paper towel or use a bend paper clip in an “L” shape.
- Slowly lower the paper into the water.
- Remove the paper from under the paper clip.
- Add some soap water to the clear water.
- What Happens?
Experiment #2
This is a neat trick! Ask friends how many quarters can go in a completely full glass of water before it overflows. The answer will generally be one or two. Then follow the steps below to prove them wrong.
- Needed materials
- 10 to 12 Quarters
- glass full of water
- Procedure
The glass should be filled to the very rim, with a slightly convex shape to the surface of the liquid. Slowly, and with a steady hand, bring the quarters one at a time to the center of the glass. Place the narrow edge of the quarter in the water and let go. (This minimizes disruption to the surface, and avoids forming unnecessary waves that can cause overflow.)
As you continue with more quarters, you will be astonished how convex the water becomes on top of the glass without overflowing!
Possible Variant: Perform this experiment with identical glasses, but use different types of coins in each glass. Use the results of how many can go in to determine a ratio of the volumes of different coins
Experiment #3 Floating Needle
Another nice surface tension trick, this one makes it so that a needle will float on the surface of a glass of water. There are two variants of this trick, both impressive in their own right.
- Needed materials
- fork (variant 1)
- piece of tissue paper (variant 2)
- sewing needle
- glass full of water
Variant 1 Trick
Place the needle on the fork, gently lowering it into the glass of water. Carefully pull the fork out, and it is possible to leave the needle floating on the surface of the water.
This trick requires a real steady hand and some practice, because you must remove the fork in such a way that portions of the needle do not get wet ... or the needle will sink. You can rub the needle between your fingers beforehand to "oil" it increase your success chances.
Variant 2 Trick
Place the sewing needle on a small piece of tissue paper (large enough to hold the needle). The needle is placed on the tissue paper. The tissue paper will become soaked with water and sink to the bottom of the glass, leaving the needle floating on the surface.
6
6a
The best materials for large bubble wands are composed of natural fibers such as cotton or wool yarn.
6b
Bamboo garden poles work well and are inexpensive--basically two sticks with a loop of yarn or cotton cording strung between them.
7
7a
It is best to use distilled water when making a bubble solution.
7b
Dawn dish washing soap seems to work best but any dish soap will do.
7c
Glycerin or corn syrup gives a bubble extra strength.
7d
Water Use good quality water that does not contain high levels of iron or minerals.
Soap When it comes to soap, Dawn dish soap seems to work the best for homemade bubble solution.
Glycerin This is the secret ingredient that gives a bubble its extra strength.
Formula # 1
- 2/3 cup (150 ml) Dawn dishwashing soap
- 1 gallon (4 liters) water
- 2 to 3 tablespoons (30-45 ml) of glycerin (available at the pharmacy or chemical supply house.)
Formula #2
- 1 cup (250ml) dish soap
- 14 cups 3.4 liters) water
- 1/4 cup (60ml) glycerin
7e
8
8a
When one bubble meets with another, the resulting union is always one of total sharing and compromise (Human beings could learn a lot from bubbles.) Since bubbles always try to minimize surface area two bubbles will merge to share a common wall. If the bubbles are the same size, this wall will be flat. If the bubbles are different sized, the smaller bubble, which always has a higher internal pressure, will bulge into the larger bubble
8b
Similar to the way we perceive the colors in a rainbow or an oil slick, we see the colors in a bubble through the reflection of interfered light waves off the inner and outer surfaces of the bubble wall.
White light is made up of all colors, all wavelengths. If one of these colors is subtracted from white light (by interference, for example) we see the complementary color.
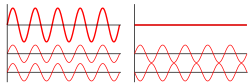
Interference: All waves including light, have a curious property. If two waves combine, the waves meet each other crest to crest, adding up and reinforcing the effect of each other or they can meet crest to trough canceling each other out so that they have no effect.
Constructive interference is when two waves are in phase. The waves come together and make a bigger wave.
Destructive interference is when two waves are out of phase and they cancel each other out.
If we were to look at a highly magnified portion of a soap bubble membrane, we would notice that light reflects off both the front (outside) and rear (inside) surfaces of the bubble. But the ray of light that reflects off the inside surface travels a longer distance than the ray which reflects from the outside surface.
When the rays recombine they get “out of phase” with each other and interfere. Given a certain thickness of the bubble wall, a certain wavelength will be canceled and its complementary color will be seen.
8c
Soap bubbles will assume the shape of least surface area possible containing a given volume. No matter what shape a bubble has initially, it will try to become a sphere. The sphere is the shape that minimizes the surface area of the structure, which makes it the shape that requires minimum energy to achieve.
Experiment
Bend pipe cleaners into several different shapes. Make as many different shapes as you can. You might want to make some square, some circles, some squares, some triangles, some heart-shaped or some even the shapes of letters. Make sure each one has a handle.
Carefully pour the bubble solution in a pie pan. Take each shaped pipe cleaner and dip it into the solution. Now start your bubble blowing.
What shapes are the Bubbles?
Record your Data.