Difference between revisions of "AY Honors/Fishes/Answer Key"
m (- Category of AYHAB) |
|||
| (230 intermediate revisions by 45 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{HonorSubpage}} |
| + | <!--{{Honor_Master|honor=Fishes|master=Naturalist|group=Domestic}}--> | ||
| + | <!--{{Honor_Master|honor=Fishes|master=Zoology|group=Domestic}}--> | ||
| + | <section begin="Body" /> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=1}} | ||
| + | <noinclude><translate><!--T:68--> | ||
| + | </noinclude> | ||
| + | <!-- 1. Name ten families of fishes. --> | ||
| + | <gallery widths="200px" perrow="3"> | ||
| + | Image:Engraulis japonica.jpg|<center>'''Engraulidae'''<br>(Anchovies)</center> | ||
| + | Image:hippocampus.jpg|<center>'''Syngnathidae'''<br>(Seahorses and pipefish)</center> | ||
| + | Image:Smallmouth bass.jpg|<center>'''Percichthyidae'''<br>(Temperate Basses)</center> | ||
| + | Image:Enneacanthus chaetodon 01.jpg|<center>'''Centrarchidae'''<br>(Sunfish)</center> | ||
| + | Image:YellowPerch.jpg|<center>'''Percidae'''<br>(Perch)</center> | ||
| + | Image:Pleuronectes platessa.jpg|<center>'''Pleuronectidae'''<br>(Righteyed Flounders)</center> | ||
| + | Image:Common carp.jpg|<center>'''Cyprinidae'''<br>(Carps and Minnows)</center> | ||
| + | Image:Molly.jpg|<center>'''Poeciliidae'''<br>(Mollies, Guppies)</center> | ||
| + | Image:Truite arc-en-ciel.jpg|<center>'''Salmonidae'''<br>(Salmon and Trout)</center> | ||
| + | Image:No work.svg | ||
| + | File:Acipenser oxyrhynchus.jpg|<center>'''Acipenseridae'''<br>(Sturgeons)</center> | ||
| + | </gallery> | ||
| − | + | <!--T:69--> | |
| − | + | <noinclude></translate></noinclude> | |
| − | == | + | {{CloseReq}} <!-- 1 --> |
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=2}} | ||
| + | <noinclude><translate></noinclude> | ||
| + | <!-- 2. Identify from pictures or personal observation ten tropical fishes.<br> a. Explain their breeding habits.<br> b. Give the habitat or country in which they are found. --> | ||
| + | ===Neon Tetra=== <!--T:70--> | ||
| + | {{Species id | ||
| + | | common_name = Neon Tetra | ||
| + | | latin_name = Paracheirodon innesi | ||
| + | | image =Neonka obecna paracheirodon innesi.jpg | ||
| + | | caption = | ||
| + | | description = The Neon Tetra has a dark olive-green back over a silver-white abdomen. The fish is characterized by an iridescent blue horizontal stripe along each side of the fish from its nose to the base of the adipose fin, and an iridescent red stripe that begins at the middle of the body and extends posteriorly to the base of the caudal fin. During the night, the color disappears as the fish rests—it reactivates once it becomes active in the morning. It grows to approximately 3 cm (1.25 in) in overall length. Sexual dimorphism is slight, the female having a slightly larger belly. | ||
| + | | range = The Neon Tetra is native to blackwater or clearwater streams in southeastern Colombia, eastern Peru, and western Brazil, including the tributaries of the Solimões. Fish are collected in warm-flowing (21–29°C) clear and blackwater streams, but never in whitewater rivers of Andean origin. | ||
| + | | reproduction = To breed Neon Tetras, place a pair of the species in a breeding tank without any light, and gradually increase the lighting until spawning occurs. Other inducers include mosquito larvae and a hardness of less than 4 degrees. Some also recommend letting the level of nitrates rise, then do at least 50% water change to simulate the fresh rain the tetras get in their natural habitat, the Amazon. It is recommended that everything you place in the aquarium be sterilized, as well as the aquarium top. Because the adults will often eat newly-hatched fry, it is best to remove them as soon as the eggs have been laid. The eggs are especially sensitive to light. Eggs will hatch within 24 hours of the laying. Fry can be fed rotifers, especially infusoria and egg yolk for 1 to 4 weeks, followed by nauplii of brine shrimp, shaved cattle liver, and formulated diets. Fry will achieve their adult coloration at approximately one month of age. Adults can spawn every two weeks. | ||
| + | }} | ||
| − | The | + | ===Afra Cichlid=== <!--T:4--> |
| + | {{Species id | ||
| + | | common_name = Afra Cichlid | ||
| + | | latin_name = Cynotilapia afra | ||
| + | | image =C afra.jpg | ||
| + | | caption = | ||
| + | | description = The afra cichlid has an elongate body typically with vertical blue and black bars. However, there are many different colorations depending on the region the fish is from, for example, the male fish from Kobwe have greenish stripes on blue-black body. They can grow up to 10 cm. Like many other cichlid from Lake Malawi, afra cichlids are mouthbrooders. Males defend boulders as their territories and feed from algae on those boulders. Females congregate in mid-water and feed from plankton. | ||
| + | | range = This fish is endemic to the central and northern parts of Lake Malawi and is found in rocky habitats. | ||
| + | | reproduction = Like many mbuna cichlids, this is a notoriously aggressive fish that should be kept in a species or mbuna tank. The best practice is to keep one male with several females. They should be provided with large spaces with plenty of hiding shelters. | ||
| + | }} | ||
| − | + | ===Angel Fish=== <!--T:5--> | |
| + | {{Species id | ||
| + | | common_name = Angel Fish | ||
| + | | latin_name = Pterophyllum spp. | ||
| + | | image =Pterophyllum altum.jpg | ||
| + | | caption = | ||
| + | | description = The three species of Pterophyllum are unusually shaped for cichlids being greatly laterally compressed, with round bodies and elongated triangular-shaped dorsal and anal fins. This body shape allows them to hide among roots and plants, often on a vertical surface. Naturally occurring angelfish are frequently striped longitudinally, colouration which provides additional camouflage. Angelfish are ambush predators and prey on small fish and macroinvertebrates. All Pterophyllum species form monogamous pairs. Eggs are generally laid on a submerged log or a flattened leaf. As is the case for other cichlids, brood care is highly developed. | ||
| + | | range = All Pterophyllum species originate from the Amazon River basin in tropical South America. | ||
| + | | reproduction = ''P. scalare'' is relatively easy to breed in the aquarium, although one of the results of generations of inbreeding is that many breeds have almost completely lost their rearing instincts resulting in the tendency of the parents to eat their young. In addition, it is very difficult to accurately identify the gender of any individual until they are nearly ready to breed. | ||
| − | + | <!--T:6--> | |
| + | Angelfish pairs form long-term relationships where each individual will protect the other from threats and potential suitors. Upon the death or removal of one of the mated pair, some breeders have experienced a total refusal of the other mate to pair up with any other angelfish; others have had more success with subsequent mates. Both parents care for the young. | ||
| − | + | <!--T:7--> | |
| + | Depending upon aquarium conditions, P. scalare reaches sexual maturity at the age of six to twelve months or more. In situations where the eggs are removed from the aquarium immediately after spawning, the pair is capable of spawning every seven to ten days. Around the age of approximately three years, spawning frequency will decrease and eventually cease. | ||
| − | + | <!--T:8--> | |
| + | When the pair is ready to spawn, they will choose an appropriate medium upon which to lay the eggs and spend one to two days picking off detritus and algae from the surface. This medium may be a broad-leaf plant in the aquarium, a flat surface such as a piece of slate placed vertically in the aquarium, a length of pipe, or even the glass sides of the aquarium. The female will deposit a line of eggs on the spawning substrate, followed by the male who will fertilize the eggs. This process will repeat itself until there are a total of 100 to up to 1200+ eggs, depending on the size and health of the female fish. The pair will take turns maintaining a high rate of water circulation around the eggs by swimming very close to the eggs and fanning the eggs with their pectoral fins. In a few days, the eggs hatch and the fry remain attached to the spawning substrate. During this period, the fry will not eat and will survive by consuming the remains of their yolk sacs. At one week, the fry will detach and become free-swimming. Successful parents will keep close watch on the eggs until they become free-swimming. At the free-swimming stage, the fry can be fed newly-hatched frozen och fresh (i.e. alive) brine shrimp (Artemia spp.). | ||
| + | }} | ||
| − | == | + | ===Discus=== <!--T:9--> |
| − | + | {{Species id | |
| − | The | + | | common_name = Discus |
| + | | latin_name = Symphysodon spp. | ||
| + | | image = Blue Discus.jpg | ||
| + | | caption = | ||
| + | | description = Like cichlids from the genus Pterophyllum, all Symphysodon species have a laterally compressed body shape. In contrast to Pterophyllum, however, extended finnage is absent giving Symphysodon a more rounded shape. It is this body shape from which their common name, “discus”, is derived. The sides of the fish are frequently patterned in shades of green, red, brown, and blue. The height and length of the grown fish are both about {{units|20–25 cm|8–10 in}}. | ||
| + | | range = Discus (''Symphysodon spp.'') are a genus of three species of freshwater cichlid fishes native to the Amazon River basin | ||
| + | | reproduction = Another characteristic of Symphysodon species is the way they care for their larvae. As for most cichlids, brood care is highly developed with both the parents caring for the young. Additionally, adult discus produce a secretion through their skin, off which the larvae live during their first few days. This behaviour has also been observed for Uaru species. | ||
| + | }} | ||
| − | + | ===Gold Barb=== <!--T:10--> | |
| + | {{Species id | ||
| + | | common_name = Gold Barb | ||
| + | | latin_name = Puntius semifasciolatus | ||
| + | | image =Gold Barb Puntius semifasciolatus 6.png | ||
| + | | caption = | ||
| + | | description = The gold barb is a medium-long barb. Adults have highly arched backs and a short pair of barbels on the upper jaw at the corners of the mouth. The back is light to reddish brown, the sides are metallic green or yellow-green, with a brassy or golden sheen below. The belly is whitish, turning orange-red in males at mating time. Females can be distinguished by their dull colors and their overall bulk. The average size of adults is {{units|7 - 8 cm|2.75 to 3 inches}}. | ||
| + | | range = Its native habitat is the Red River basin in southeast China. | ||
| + | | reproduction = An egg-scatterer, adult barbs will spawn around a hundred eggs. This breeding occurs at the first light in the early morning. | ||
| + | }} | ||
| − | + | ===Guppy=== <!--T:11--> | |
| + | {{Species id | ||
| + | | common_name = Guppy | ||
| + | | latin_name = Poecilia reticulata | ||
| + | | image =Guppy-Male-and-Female.JPG | ||
| + | | caption = | ||
| + | | description = The guppy (Poecilia reticulata) is one of the most popular freshwater aquarium fish species in the world. It is a small member of the Poecilidae family (females 4-6 centimeters long, males 2½–3½ centimeters long) and like all other members of the family, is live-bearing. | ||
| + | | range = Guppies are native to Trinidad and parts of South America, specifically Antigua and Barbuda, Barbados, Brazil, Guyana, Netherlands Antilles, Trinidad and Tobago, the US Virgin Islands, and Venezuela. | ||
| + | | reproduction = Guppies are highly prolific livebearers. The gestation period of a guppy is 22-30 days, with an average of 28 days. After the female guppy is inseminated, a dark area near the anus, known as the gravid spot, will enlarge and darken. Guppies prefer water temperatures of about {{units|28 °C|82 °F}} for reproduction. The female guppy drops of between 2-100 fry, typically ranging between 30 and 60. After giving birth, the female is ready for conception again within only a few hours. | ||
| + | }} | ||
| − | == | + | ===Molly=== <!--T:12--> |
| − | + | {{Species id | |
| − | + | | common_name = Molly | |
| + | | latin_name = Poecilia sphenops | ||
| + | | image =Black molly.jpg | ||
| + | | caption = | ||
| + | | description = This species is one of the most well-known aquarium fishes and nearly as easy to keep and prolific as guppies (for optimal health and breeding success, they demand fresh vegetable food like algae). There are several other popular breeds, like the golden molly nicknamed "24 karat", or the balloon molly, which however has a deformed spine and a decreased lifespan due to the associated health problems. Also, breeds with altered caudal fin structures like lyretails exist. The wild form is in fact quite rarely kept, as it has a rather plain silvery coloration suffused with brown and green hues. If given good care with ample sunlight, high water temperatures and fresh vegetables, they will, however, prove charming fish who make up for their somewhat plain coloration with their lively behavior. | ||
| + | | range = Mollies inhabit the coastal brackish and marine waters of Mexico. | ||
| + | | reproduction = Fertilization is internal and is accomplished by means of highly modified fin elements within the anal fin of males that form a structure known as the gonopodium. Sailfin mollies produce broods of 10-140 live young, depending upon maturity and size, and females may store sperm long after the demise of their relatively short-lived mates. The gestation period for this species is approximately 3-4 weeks, depending upon temperature, and a single female may give birth on multiple occasions throughout the year. Although sex ratios of the broods are balanced, adult populations tend to be largely female as males appear to suffer higher rates of mortality due to a greater susceptibility to predators and disease as a consequence of their brighter colours and a life devoted to frenzied breeding. There is no parental care exhibited by this species. | ||
| + | }} | ||
| − | + | ===Betta=== <!--T:13--> | |
| − | + | <!--T:14--> | |
| + | {{Species id | ||
| + | | common_name = Betta | ||
| + | | latin_name = Betta | ||
| + | | image =Betta albimarginata 060311 8.jpg | ||
| + | | caption = | ||
| + | | description = Betta Bleeker, 1850 is a large genus of small, often colorful, freshwater ray-finned fishes. All the Betta species are small fishes, but they vary considerably in size, ranging from under 2.5 cm (1 inch) total length in B. chanoides to 14 cm (5.5 inches) in the Akar betta (B. akarensis).[1] | ||
| − | === | + | Bettas are anabantoids, which means they can breathe atmospheric air thanks to a unique organ called the labyrinth. This accounts for their ability to thrive in low-oxygen water conditions that would kill most other fish, such as rice paddies, slow-moving streams, drainage ditches, and large puddles. |
| − | The | + | | range = The Siamese fighting fish (Betta splendens) is one of the most popular species of freshwater aquarium fish. It is native to the Mekong river basin in Southeast Asia. |
| + | | reproduction = The various bettas can be divided into two groups, based on their spawning behavior: some build bubble nests, like B. splendens, while others are mouthbrooders, like B. picta. The mouthbrooding species are sometimes called "pseudo bettas". | ||
| + | }} | ||
| + | ===Goby=== <!--T:15--> | ||
| + | {{Species id | ||
| + | | common_name = Goby | ||
| + | | latin_name = Elacatinus | ||
| + | | image =Elacatinus evelynae.jpg | ||
| + | | caption = | ||
| + | | description = Neon gobies are very small, torpedo-shaped fish. Although sizes vary slightly by species, they are generally about {{units|2.5 cm|1 inch}} long. They have dark bodies with iridescent stripes (the color of which varies by species) running from the tip of the nose to the base of the caudal fin. Like all gobies, their dorsal fin is split in two, the anterior dorsal fin being rounded like that of a clownfish and the posterior dorsal fin being relatively flat. The anal fin lines up with the posterior dorsal fin and is of similar shape. The pectoral fins are nearly circular, and, like all other fins, transparent. | ||
| + | |||
| + | They are well-documented cleaner fish, setting up stations where often much larger fish (sometimes even fish who would normally eat the gobies) come to have the gobies eat their small external parasites. This is an excellent example of symbiosis – the cleaned fish are healthier and the gobies have not only an excellent food source but also relative protection from potential predators. | ||
| + | | range = Neon gobies are native to the tropical reefs of the Gulf of Mexico, from Texas to Belize, where they live primarily in the rocks. | ||
| + | | reproduction = If kept in pristine conditions and fed well neon gobies will readily spawn in home aquaria. A species or breeding tank is required, as the fry are small and will be eaten by most other fish. The gobies are sexually dimorphic, but the difference is not easy to ascertain so they are normally kept in large groups to ensure a balance of sexes. They will lay their eggs on any hard surface along the bottom, and the fry, which feed on small rotifers or other microscopic organisms, are fully developed within a month. The average lifespan for a neon goby is approximately a year to a year and a half. | ||
| + | }} | ||
| + | ===Green Swordtail=== <!--T:16--> | ||
| + | {{Species id | ||
| + | | common_name = Green Swordtail | ||
| + | | latin_name = Xiphophorus hellerii | ||
| + | | image =Xiphophorus helleri 02.jpg | ||
| + | | caption = | ||
| + | | description = The male green swordtail grows to a maximum overall length of {{units|14cm|5.5i}} and the female to {{units|16 cm|6.3 in}}. The name "swordtail" is commonly but mistakenly believed to be derived form the elongated lower lobe of the male's caudal fin (tailfin), but is actually derived from the sword shaped anal fin of the male. Sexual dimorphism is moderate, with the female being larger than the male but lacking the "sword". The wild form is olive green in color, with a red or brown lateral stripe and speckles on the dorsal and, sometimes, caudal fins. The male's "sword" is yellow, edged in black below. Captive breeding has produced many color varieties, including black, red, and many patterns thereof, for the aquarium hobby. | ||
| + | | range = It is native to an area of North and Central America stretching from Veracruz, Mexico, to northwestern Honduras. | ||
| + | | reproduction = The males' elongated caudal fins have been found to significantly affect their chances at mating. The presence of a well-endowed male spurs the maturity of females while it inhibits the maturity of juvenile males in the vicinity as the well-endowed male. | ||
| + | }} | ||
| + | |||
| + | <!--T:71--> | ||
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 2 --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=3}} | ||
| + | <noinclude><translate></noinclude> | ||
| + | <!-- 3. Identify from pictures or personal observation ten fishes native to your own country. Explain their feeding and breeding habits. --> | ||
| + | The fish presented here are native to the United States and Canada. | ||
| + | ===Brook Trout=== <!--T:72--> | ||
| + | {{Species id | ||
| + | | common_name = Brook Trout | ||
| + | | latin_name = Salvelinus fontinalis | ||
| + | | image =Salvelinus fontinalis.jpg | ||
| + | | caption = Brook Trout | ||
| + | | description = The brook trout is of dark green to brown basic coloration with a distinctive marbled pattern (called vermiculations) of lighter shades across the flanks and back and extending at least to the dorsal fin, and often to the tail. There is a distinctive sprinkling of red dots, surrounded by blue haloes, along the flank. The belly and lower fins are reddish in color, the latter with white leading edges. Often the belly, particularly of the males, becomes very red or orange when the fish are spawning. The species reaches a maximum recorded length of 86 cm (33 in) and a maximum recorded weight of 9.4 kg (21 lb). It can reach at least seven years of age, with reports of 15-year-old specimens observed in California habitats to which the species has been introduced. | ||
| + | | range = The brook trout is native to small streams, creeks, lakes, and spring ponds. Some brook trout are anadromous. It is native to a wide area of eastern North America but increasingly confined to higher elevations southward in the Appalachian Mountains to northern Georgia, Canada from the Hudson Bay basin east, the Great Lakes–Saint Lawrence system, and the upper Mississippi River drainage as far west as eastern Iowa. | ||
| + | | reproduction = Individuals normally spend their entire life in fresh water, but some — colloquially called "salters" or "sea run" — may spend up to three months at sea in the spring, not straying more than a few kilometers from the river mouth. The fish return upstream to spawn in the late summer or autumn. The female constructs a depression in a location in the stream bed, sometimes referred to as a "redd", where groundwater percolates upward through the gravel. One or more males approaches the female, fertilizing the eggs as the female expresses them. The eggs are slightly more dense than water. The female then buries the eggs in a small gravel mound. The eggs hatch in approximately 100 days. | ||
| + | | diet = Its diverse diet includes crustaceans, frogs and other amphibians, insects, molluscs, smaller fish, and even small aquatic mammals such as voles. | ||
| + | }} | ||
| + | ===Rainbow Trout=== | ||
| + | {{Species id | ||
| + | | common_name = Rainbow Trout | ||
| + | | latin_name = Oncorhynchus mykiss | ||
| + | | image =Oncorhynchus mykiss mid res 150dpi.jpg | ||
| + | | caption = | ||
| + | | description = The rainbow trout are unusual in that there are two forms which sometimes share the same habitat. The anadromous (sea-going) form called "steelhead" migrate to the ocean, though they must return to fresh water to reproduce. The freshwater form is called "rainbow trout", based on the broad red band along their sides. Steelhead are exactly the same species as rainbow trout. However, the difference is anadromy. After going to sea, their color changes, including loss of the red band. They stay at sea for 1-4 years, and return to fresh water to spawn. Rainbows stay in fresh water their whole lives. Rainbows and steelhead occur in well-oxygenated lakes and streams where the temperature normally doesn't rise above 12°C in summer. | ||
| + | |||
| + | <!--T:18--> | ||
| + | Rainbows range from 12 to 36 inches in length. Steelhead grow longer, ranging from 50 to 122 cm (20 to 48 inches) in length. Steelhead range in weight from 2.5 kg to 10 kg (5.5 - 22 pounds). | ||
| + | | range = The rainbow trout is a species of salmonid native to tributaries of the Pacific Ocean in Asia and North America as well as much of the central, western, eastern, and especially the northern portions of the United States. | ||
| + | | reproduction = Unlike other Pacific Salmon, rainbow trout and steelhead do not necessarily die after spawning (they may spawn as many as four times). | ||
| + | | diet =Rainbow trout have a varied diet. They are predators, eating any smaller fish from nearly the time they are born. Insects make up a large portion of the diet, along with crayfish and other crustaceans, some lake dwelling species may become planktonic feeders. Trout of all ages will eat nearly anything they can grab, in contrast with the legendary, selective nature the fish often gets. They are near the top of the food chain in most freshwater environments. However they are lower on the rung of other freshwater predators such as pike, muskie, lake trout, and chinook salmon. Rainbows will take fish up to and over 1/3 of their length and larger. | ||
| + | }} | ||
| − | + | ===Northern Pike=== <!--T:19--> | |
| + | {{Species id | ||
| + | | common_name = Northern Pike | ||
| + | | latin_name = Esox lucius | ||
| + | | image =Esox Lucius.JPG | ||
| + | | image_2 = Esox lucius1.jpg | ||
| + | | caption = | ||
| + | | description = Northern pike are most often olive, shading into yellow to white along the belly. The flank is marked with short, light barlike spots and there are a few to many dark spots on the fins. The lower half of the gill cover lacks scales and they have large sensory pores on their head and on the underside of the lower jaw which are part of the lateral line system. Unlike the similar-looking and closely related muskellunge, the northern pike has light markings on a dark body background and fewer than six sensory pores on the underside of each side of the lower jaw. | ||
| + | | range = E. lucius is found throughout the northern hemisphere, including Russia, Europe, the British Isles, and North America. | ||
| + | | reproduction = The Northern Pike spawns beneath the ice in the Spring. A female is followed by four or five smaller males as she seeks vegetative cover in the shallows. When the eggs are released and fertilized, the stick to the vegetation where they are abandoned by the parents. | ||
| + | | diet =Pike are typical ambush predators; they lie in wait for prey, holding perfectly still for long periods and then exhibit remarkable acceleration as they strike. The fish has a distinctive habit of catching its prey sideways in the mouth, killing or immobilizing it with its sharp teeth, and then turning the prey lengthwise to swallow it. It eats mainly fish, but on occasion water voles and ducklings have also been known to fall prey to pike. Pike will aggressively strike at any fish in the vicinity, even at other pike. Young pike have been found dead from choking on a pike of a similar size. Northern pike also feed on frogs, insects and leeches. It has often been suggested that pike optimally forage on prey that are from 25 to 35% of their body length. Also on rare occasions pike have been reported to have eaten young bald eagles. | ||
| + | }} | ||
| − | + | ===Bluegill=== <!--T:20--> | |
| + | {{Species id | ||
| + | | common_name = Bluegill | ||
| + | | latin_name = Lepomis macrochirus | ||
| + | | image =Lepomis macrochirus photo.jpg | ||
| + | | caption = | ||
| + | | description = The Bluegill is a species of freshwater fish sometimes referred to as bream, brim, or copper nose. The bluegill's most notable feature is the blue or black "ear", actually an extension of the gill cover called the opercular flap. Its name, however, comes from the bright blue edging visible on its gill rakers. It can be distinguished from similar species by the (not always pronounced) vertical bars along its flanks. The bluegill grows to a maximum overall length of approximately 40 cm (16 in). | ||
| + | | range = The bluegill is native to a wide area of North America, from Québec to northern Mexico, and has been widely transplanted to stock game fish for anglers. | ||
| + | | reproduction = These fish spawn in June in nests in the shallows. During this period males assume a very bold coloration, as they are guarding their nests. An interesting aspect of their biology is that some males assume the coloration of the female fish so that the nest-guarding males won't show aggression towards them. Then these "sneaker" males enter nests and spawn. | ||
| + | | diet = The natural diet of the bluegill consists largely of small invertebrates and very small fish. | ||
| + | }} | ||
| − | + | ===Smallmouth Bass=== <!--T:21--> | |
| + | {{Species id | ||
| + | | common_name = Smallmouth Bass | ||
| + | | latin_name = Micropterus dolomieu | ||
| + | | image =Micropterus dolomieu2.jpg | ||
| + | | caption = | ||
| + | | description = The smallmouth bass is generally green with dark vertical bands rather than a horizontal band along the side. There are 13-15 soft rays in the dorsal fin. The upper jaw of smallmouth bass does not extend beyond the back of the eye. | ||
| + | | range = The smallmouth bass is native to the upper and middle Mississippi River basin, the Saint Lawrence River–Great Lakes system, and up into the Hudson Bay basin. | ||
| + | | reproduction = The female can lay up to 21,000,000 eggs, which are guarded by the male in his nest. | ||
| + | | diet =Carnivorous, its diet comprises crayfish, insects, and smaller fish, the young also feeding on zooplankton. | ||
| + | }} | ||
| − | The | + | ===Largemouth Bass=== <!--T:22--> |
| + | {{Species id | ||
| + | | common_name = Largemouth Bass | ||
| + | | latin_name = Micropterus salmoides | ||
| + | | image =Micropterus salmoides 2.jpg | ||
| + | | caption = | ||
| + | | description = The largemouth is marked by a series of dark, sometimes black, blotches forming a jagged horizontal stripe along each flank. The upper jaw of a largemouth bass extends beyond the rear edge of the eye. | ||
| + | | range = Native to the central and southeastern United States, the largemouth bass has been introduced throughout most of the United States and Canada.<ref>http://www.bio.umass.edu/biology/conn.river/smlgbass.html</ref> | ||
| + | | reproduction = Spawning occurs in shallow areas of lakes and ponds in the spring. Males arrive first, selecting a territory on fine gravel, coarse sand or among sparse vegetation. The male will slightly clear, using his fins, a shallow depression into which he will entice a female to deposit her eggs. Females can lay up to a million eggs during each season. The male guards the embryos until the larvae hatch and then will continue to guard the "fry" until they disperse from the nest. During the guarding period, the male ferociously attacks any potential predators that approach too closely. | ||
| + | | diet =The juvenile largemouth bass consumes mostly zooplankton and insects. Adults consume small fish, crayfish, and frogs. Prey items can be as large as 25 to 35% of the bass's body length. Largemouth bass have even been reported to take small birds, small mammals, such as mice and rats and small snakes. | ||
| + | }} | ||
| − | + | ===White Perch=== <!--T:23--> | |
| + | {{Species id | ||
| + | | common_name = White Perch | ||
| + | | latin_name = Morone americana | ||
| + | | image =White perch GLERL.jpg | ||
| + | | caption = | ||
| + | | description = Generally silvery-white in color, hence the name, it has been reported up to 49.5 cm in length and weighing 2.2 kg. | ||
| + | | range = Although favoring brackish waters, it is also found in fresh water and coastal areas from the St. Lawrence River and Lake Ontario south to the Pee Dee River in South Carolina. | ||
| + | | reproduction = White perch are a prolific species. The female can deposit over 140,000 eggs in a spawning session, lasting just over a week. Several males will often attend a spawning female, and each may fertilize a portion of her eggs. The young hatch within 1 to 6 days of fertilization. | ||
| + | | diet =White perch are known to eat the eggs of many species native to the Great Lakes, such as walleye and other true perches. At times, fish eggs are 100% of its diet. | ||
| + | }} | ||
| − | === | + | ===Black Crappie=== <!--T:24--> |
| − | + | {{Species id | |
| + | | common_name = Black Crappie | ||
| + | | latin_name = Pomoxis nigromaculatus | ||
| + | | image =Pomoxis nigromaculatus1.jpg | ||
| + | | caption = | ||
| + | | description = The black crappue is most accurately identified by the seven or eight spines on its dorsal fin. | ||
| + | | range = The crappie is native throughout the eastern half of Canada and the United States, and has been widely introduced in the west as well. As of 2005, populations existed in all of the lower 48 states of the United States. | ||
| + | | reproduction = The breeding season varies by location, due to the species’ great range; breeding temperature is 14‒20 °C (58‒68 °F) and spawning occurs between April and June. Spawning occurs in a nest built by the male, who guards the eggs and young. | ||
| + | | diet =Adult crappies feed predominantly on smaller species, including the young of their own predators (which include the northern pike, muskellunge, and walleye). They have diverse diets, however, including zooplankton, insects, and crustaceans | ||
| + | }} | ||
| − | + | ===Striped Bass=== <!--T:25--> | |
| + | {{Species id | ||
| + | | common_name = Striped Bass | ||
| + | | latin_name = Morone saxatilis | ||
| + | | image =StripedBass.JPG | ||
| + | | caption = | ||
| + | | description = The striped bass is a typical member of the Moronidae family in shape, having a streamlined, silvery body marked with longitudinal dark stripes running from behind the gills to the base of the tail. Maximum size is 200 cm (6.6 ft) and maximum scientifically recorded weight 57 kg (125 US pounds). Striped bass are believed to live for up to 30 years. | ||
| + | | range = Striped bass are found along the Atlantic coastline of North America from the St. Lawrence River into the Gulf of Mexico to approximately Louisiana. They are anadromous fish that migrate between fresh and salt water. Spawning takes place in freshwater. | ||
| + | | reproduction = Striped bass spawn in freshwater and spend their adult lives in saltwater (i.e., it is anadromous). They can also live exclusively in freshwater and currently flourish in several inland water bodies. | ||
| + | | diet = Striped bass are primarily nocturnal feeders, eating insects, crustaceans, and other fish. | ||
| + | }} | ||
| − | ==== | + | ===Haddock=== <!--T:26--> |
| − | + | {{Species id | |
| + | | common_name = Haddock | ||
| + | | latin_name = Melanogrammus aeglefinus | ||
| + | | image =Melanogrammus aeglefinus.jpg | ||
| + | | caption = | ||
| + | | description = The haddock is easily recognized by a black lateral line running along its white side, not to be confused with pollock which has the reverse, ie white line on black side, and a distinctive dark blotch above the pectoral fin. They grow up to {{units|1.1 meters|43 inches}} in length. | ||
| + | | range = The haddock or offshore hake is a marine fish distributed on both sides of the North Atlantic. | ||
| + | | reproduction = Spawning occurs between January and June, peaking during late March and early April. The most important spawning grounds are in the waters off middle Norway near southwest Iceland, and Georges Bank. An average-sized female produces approximately 850,000 eggs, and larger females are capable of producing up to 3 million eggs each year. | ||
| + | | diet =Haddock feed primarily on small invertebrates, although larger members of the species may occasionally consume fish. | ||
| + | }} | ||
| − | + | <!--T:73--> | |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 3 --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=4}} | ||
| + | <noinclude><translate><!--T:74--> | ||
| + | </noinclude> | ||
| + | <!-- 4. Define the following parts of a fish: --> | ||
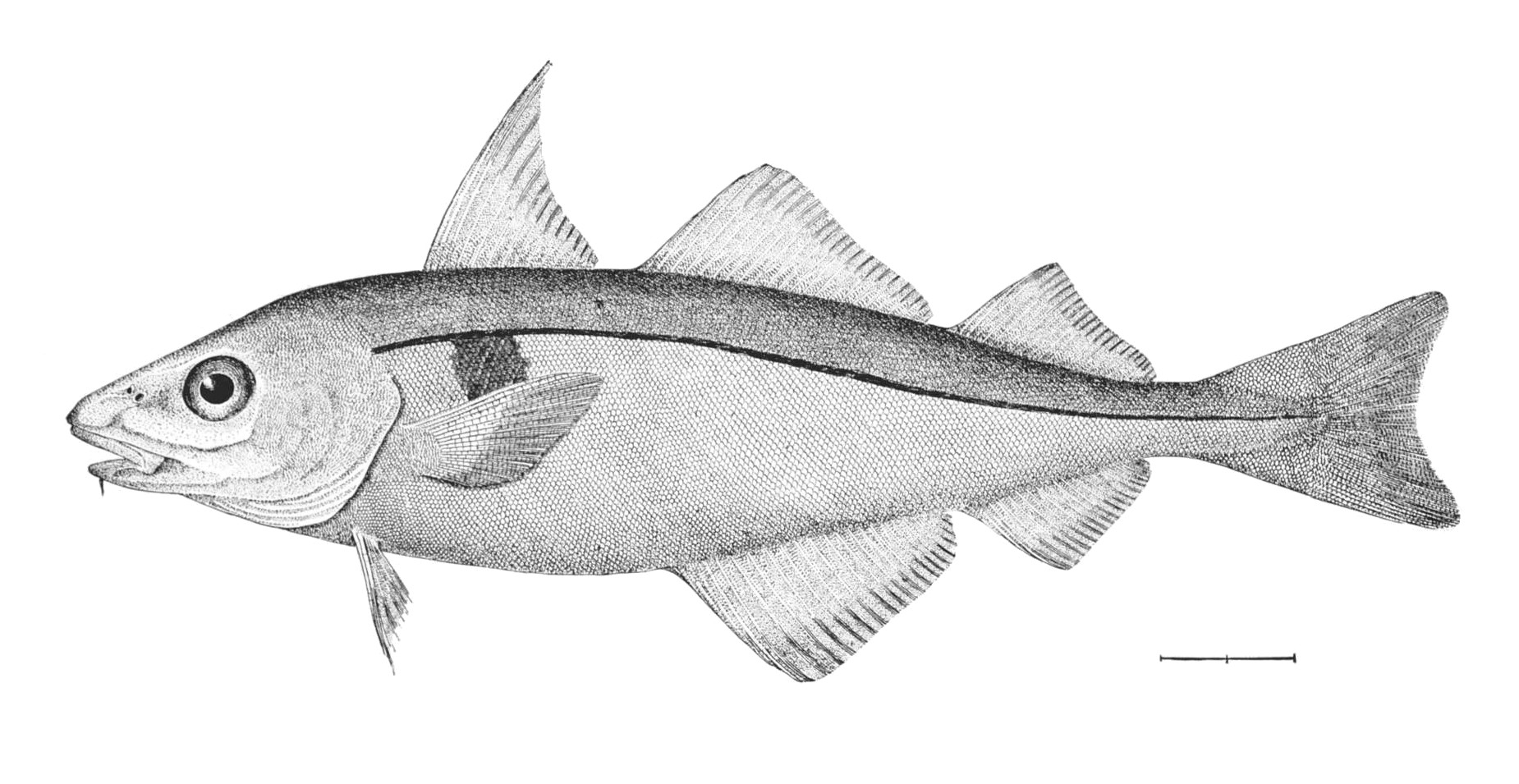
| − | == | + | <!--T:75--> |
| + | [[Image:Lampanyctodes hectoris (Hector's lanternfish)2.png|thumb|350px|''Lampanyctodes hectoris'' <br><small> | ||
| + | (1) - operculum (gill cover), (2) - lateral line, (3) - dorsal fin, (4) - adipose fin, (5) - caudal peduncle, (6) - caudal fin, (7) - anal fin, (8) - photophores, (9) - pelvic fins (paired), (10) - pectoral fins (paired)</small>]] | ||
| + | <noinclude></translate></noinclude> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=4a}} <!--T:28--> | ||
| + | <noinclude><translate><!--T:76--> | ||
| + | </noinclude> | ||
| + | A dorsal fin is a fin located on the backs of fishes, whales, dolphins and porpoises, as well as the (extinct) ichthyosaurs. Its main purpose is to stabilize the animal against rolling and assist in sudden turns. Some animals have developed dorsal fins with protective functions, such as spines or venom. Many catfish can lock the leading ray of the dorsal fin in an extended position to discourage predation or to wedge themselves into a crevice. | ||
| + | Dorsal fins come in a variety of shapes and sizes. | ||
| − | + | <!--T:77--> | |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 4a --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=4b}} <!--T:29--> | ||
| + | <noinclude><translate><!--T:78--> | ||
| + | </noinclude> | ||
| + | The paired pectoral fins are located on each side, usually just behind the operculum, and are homologous to the forelimbs of tetrapods. A peculiar function of pectoral fins, highly developed in some fish, is the creation of the dynamic lifting force that assists, e.g., sharks, in maintaining depths and enables the flight for flying fish. | ||
| − | + | <!--T:79--> | |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 4b --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=4c}} <!--T:30--> | ||
| + | <noinclude><translate><!--T:80--> | ||
| + | </noinclude> | ||
| + | The paired pelvic or ventral fins are located ventrally below the pectoral fins. They are homologous to the hindlimbs of tetrapods. | ||
| − | + | <!--T:81--> | |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 4c --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=4d}} <!--T:31--> | ||
| + | <noinclude><translate><!--T:82--> | ||
| + | </noinclude> | ||
| + | The anal fin is located on the ventral surface behind the anus. This fin is used to stabilize the fish while swimming. | ||
| − | + | <!--T:83--> | |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 4d --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=4e}} <!--T:32--> | ||
| + | <noinclude><translate><!--T:84--> | ||
| + | </noinclude> | ||
| + | The caudal fin is the tail fin, located at the end of the caudal peduncle. | ||
| − | The | + | <!--T:85--> |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 4e --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=4f}} <!--T:33--> | ||
| + | <noinclude><translate><!--T:86--> | ||
| + | </noinclude> | ||
| + | The lateral line is a sense organ used to detect movement and vibration in the surrounding water. It consists of a line of receptors running along each side of the fish. | ||
| − | = | + | <!--T:87--> |
| − | + | <noinclude></translate></noinclude> | |
| − | + | {{CloseReq}} <!-- 4f --> | |
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=4g}} <!--T:34--> | ||
| + | <noinclude><translate><!--T:88--> | ||
| + | </noinclude> | ||
| + | The operculum of a bony fish is the hard bony flap covering and protecting the gills. In most fish, the rear edge of the operculum roughly marks the division between the head and the body. The operculum is composed of four bones; the opercle, preopercle, interopercle, and subopercle. The morphology of this anatomical feature varies greatly between species. For example, the bluegill (Lepomis macrochirus) has a posteriorly and dorsally oriented rounded extension with a small black splotch present. In some species, the operculum can push water from the buccal cavity through the gills. | ||
| + | For some fish, the operculum is vital in obtaining oxygen. It opens as the mouth closes, causing the pressure inside the fish to drop. Water then flows towards the lower pressure across the fish's gill lamellae, allowing some oxygen to be absorbed from the water. | ||
| + | Cartilaginous fishes do not have an operculum. Without an operculum, other methods of getting water to the gills are required, such as ventilation. | ||
| − | + | <!--T:89--> | |
| − | + | <noinclude></translate></noinclude> | |
| − | + | {{CloseReq}} <!-- 4g --> | |
| − | + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=4h}} <!--T:35--> | |
| − | + | <noinclude><translate><!--T:90--> | |
| − | + | </noinclude> | |
| − | + | The head may have several fleshy structures known as barbels, which may be very long and resemble whiskers. | |
| − | == | + | <!--T:91--> |
| − | + | <noinclude></translate></noinclude> | |
| + | {{CloseReq}} <!-- 4h --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=4i}} <!--T:36--> | ||
| + | <noinclude><translate><!--T:92--> | ||
| + | </noinclude> | ||
| + | The gas bladder, or swim bladder, is an internal organ that contributes to the ability of a fish to control its buoyancy, and thus to stay at the current water depth, ascend, or descend without having to waste energy in swimming. It is often absent in fast swimming fishes such as the Tuna and Mackerel families. | ||
| − | + | <!--T:93--> | |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 4i --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=4j}} <!--T:37--> | ||
| + | <noinclude><translate><!--T:94--> | ||
| + | </noinclude> | ||
| + | The gills, located under the operculum, are a respiratory organ for the extraction of oxygen from water and for the excretion of carbon dioxide. They are not usually visible, but can be seen in some species e.g. the frilled shark. | ||
| − | + | <!--T:95--> | |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 4j --> | ||
| + | {{CloseReq}} <!-- 4 --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=5}} | ||
| + | <noinclude><translate><!--T:96--> | ||
| + | </noinclude> | ||
| + | <!-- 5. State briefly the proper care and feeding of fishes of: <br>a. Tropical zone <br>b. Temperate zone. --> | ||
| + | Ideal aquarium ecology reproduces the balance found in nature in the closed system of an aquarium. In practice it is virtually impossible to maintain a perfect balance. As an example, a balanced predator-prey relationship is nearly impossible to maintain in even the largest of aquaria. Typically, an aquarium keeper must take steps to maintain balance in the small ecosystem contained in his aquarium. | ||
| − | + | <!--T:39--> | |
| − | + | Approximate balance is facilitated by large volumes of water. Any event that perturbs the system pushes an aquarium away from equilibrium; the more water that is contained in a tank, the easier such a systemic shock is to absorb, as the effects of that event are diluted. For example, the death of the only fish in a three U.S. gallon tank (11 L) causes dramatic changes in the system, while the death of that same fish in a 100 U.S. gallon (400 L) tank with many other fish in it represents only a minor change in the balance of the tank. For this reason, hobbyists often favor larger tanks when possible, as they are more stable systems requiring less intensive attention to the maintenance of equilibrium. | |
| − | + | <!--T:40--> | |
| + | There are a variety of nutrient cycles that are important in the aquarium. Dissolved oxygen enters the system at the surface water-air interface or through the actions of an air pump. Carbon dioxide escapes the system into the air. The phosphate cycle is an important, although often overlooked, nutrient cycle. Sulfur, iron, and micronutrients also cycle through the system, entering as food and exiting as waste. Appropriate handling of the nitrogen cycle, along with supplying an adequately balanced food supply and considered biological loading, is usually enough to keep these other nutrient cycles in approximate equilibrium. | ||
| + | ===Water conditions===The solute content of water is perhaps the most important aspect of water conditions, as total dissolved solids and other constituents can dramatically impact basic water chemistry, and therefore how organisms are able to interact with their environment. Salt content, or salinity, is the most basic classification of water conditions. An aquarium may have freshwater (salinity below 0.5 PPT), simulating a lake or river environment; brackish water (a salt level of 0.5 to 30 PPT), simulating environments lying between fresh and salt, such as estuaries; and salt water or seawater (a salt level of 30 to 40 PPT), simulating an ocean or sea environment. Rarely, even higher salt concentrations are maintained in specialized tanks for raising brine organisms. | ||
| − | + | <!--T:41--> | |
| + | Several other water characteristics result from dissolved contents of the water, and are important to the proper simulation of natural environments. The pH of the water is a measure of the degree to which it is alkaline or acidic. Saltwater is typically alkaline, while the pH of fresh water varies more. Hardness measures overall dissolved mineral content; hard or soft water may be preferred. Hard water is usually alkaline, while soft water is usually neutral to acidic. Dissolved organic content and dissolved gases content are also important factors. | ||
| − | + | <!--T:42--> | |
| + | Home aquarists typically use modified tap water supplied through their local water supply network to fill their tanks. Because of the chlorine used to disinfect drinking water supplies for human consumption, straight tap water cannot be used. In the past, it was possible to "condition" the water by simply letting the water stand for a day or two, which allows the chlorine time to dissipate. However, chloramine is now used more often as it is much stabler and will not leave the water as readily. Additives formulated to remove chlorine or chloramine are often all that is needed to make the water ready for aquarium use. Brackish or saltwater aquaria require the addition of a mixture of salts and other minerals, which are commercially available for this purpose. | ||
| − | + | <!--T:43--> | |
| + | More sophisticated aquarists may make other modifications to their base water source to modify the water's alkalinity, hardness, or dissolved content of organics and gases, before adding it to their aquaria. This can be accomplished by a range of different additives, such as sodium bicarbonate to raise pH. Some aquarists will even filter or purify their water prior to adding it to their aquarium. There are two processes used for that: deionization or reverse osmosis. In contrast, public aquaria with large water needs often locate themselves near a natural water source (such as a river, lake, or ocean) in order to have easy access to a large volume of water that does not require much further treatment. | ||
| − | + | <!--T:44--> | |
| − | + | The temperature of the water forms the basis of one of the two most basic aquarium classifications: tropical vs. cold water. Most fish and plant species tolerate only a limited range of water temperatures: Tropical or warm water aquaria, with an average temperature of about 25 °C (77 °F), are much more common, and tropical fish are among the most popular aquarium denizens. Cold water aquaria are those with temperatures below what would be considered tropical; a variety of fish are better suited to this cooler environment. More importantly than the temperature range itself is the consistency in temperature; most organisms are not accustomed to sudden changes in temperatures, which could cause shock and lead to disease. Water temperature can be regulated with a combined thermometer and heater unit (or, more rarely, with a cooling unit). | |
| − | + | <!--T:45--> | |
| + | Water movement can also be important in accurately simulating a natural ecosystem. Aquarists may prefer anything from still water up to swift simulated currents in an aquarium, depending on the conditions best suited for the aquarium's inhabitants. Water movement can be controlled through the use of aeration from air pumps, powerheads, and careful design of internal water flow (such as location of filtration system points of inflow and outflow). | ||
| − | == | + | ===Nitrogen cycle=== <!--T:46--> |
| − | + | Of primary concern to the aquarist is management of the biological waste produced by an aquarium's inhabitants. Fish, invertebrates, fungi, and some bacteria excrete nitrogen waste in the form of ammonia (which will convert to ammonium, in acidic water) and must then pass through the nitrogen cycle. Ammonia is also produced through the decomposition of plant and animal matter, including fecal matter and other detritus. Nitrogen waste products become toxic to fish and other aquarium inhabitants at high concentrations. | |
| + | ====The Process==== | ||
| + | A well-balanced tank contains organisms that are able to metabolize the waste products of other aquarium residents. The nitrogen waste produced in a tank is metabolized in aquaria by a type of bacteria known as nitrifiers (genus Nitrosomonas). Nitrifying bacteria capture ammonia from the water and metabolize it to produce nitrite. Nitrite is also highly toxic to fish in high concentrations. Another type of bacteria, genus Nitrospira, converts nitrite into nitrate, a less toxic substance to aquarium inhabitants. (Nitrobacter bacteria were previously believed to fill this role, and continue to be found in commercially available products sold as kits to "jump start" the nitrogen cycle in an aquarium. While biologically they could theoretically fill the same niche as Nitrospira, it has recently been found that Nitrobacter are not present in detectable levels in established aquaria, while Nitrospira are plentiful.) This process is known in the aquarium hobby as the nitrogen cycle. | ||
| − | + | In addition to bacteria, aquatic plants also eliminate nitrogen waste by metabolizing ammonia and nitrate. When plants metabolize nitrogen compounds, they remove nitrogen from the water by using it to build biomass. However, this is only temporary, as the plants release nitrogen back into the water when older leaves die off and decompose. | |
| + | ====Maintaining the Tank==== <!--T:47--> | ||
| + | Although informally called the nitrogen cycle by hobbyists, it is in fact only a portion of a true cycle: nitrogen must be added to the system (usually through food provided to the tank inhabitants), and nitrates accumulate in the water at the end of the process, or become bound in the biomass of plants. This accumulation of nitrates in home aquaria requires the aquarium keeper to remove water that is high in nitrates, or remove plants which have grown from the nitrates. | ||
| − | + | <!--T:48--> | |
| + | Aquaria kept by hobbyists often do not have the requisite populations of bacteria needed to detoxify nitrogen waste from tank inhabitants. This problem is most often addressed through two filtration solutions: Activated carbon filters absorb nitrogen compounds and other toxins from the water, while biological filters provide a medium specially designed for colonization by the desired nitrifying bacteria. Activated carbon and other substances, such as ammonia absorbing resins, will stop working when their pores get full, so these components have to be replaced with fresh stocks constantly. | ||
| − | + | <!--T:49--> | |
| + | New aquaria often have problems associated with the nitrogen cycle due to insufficient number of beneficial bacteria, known as the "New Tank Syndrome". Therefore, new tanks have to be "matured" before stocking them with fish. There are three basic approaches to this: the fishless cycle, the silent cycle, and slow growth. | ||
| − | + | <!--T:50--> | |
| − | + | No fish are kept in a tank undergoing a fishless cycle. Instead, small amounts of ammonia are added to the tank to feed the bacteria being cultured. During this process, ammonia, nitrite, and nitrate levels are tested to monitor progress. The silent cycle is basically nothing more than densely stocking the aquarium with fast-growing aquatic plants and relying on them to consume the nitrogen, allowing the necessary bacterial populations time to develop. According to anecdotal reports of aquarists specializing in planted tanks, the plants can consume nitrogenous waste so efficiently that the spikes in ammonia and nitrite levels normally seen in more traditional cycling methods are greatly reduced, if they are detectable at all. More commonly slow growth entails slowly increasing the population of fish over a period of 6 to 8 weeks, giving bacteria colonies time to grow and stabilize with the increase in fish waste. | |
| − | + | The largest bacterial populations are found in the filter; efficient filtration is vital. Sometimes, a vigorous cleaning of the filter is enough to seriously disturb the biological balance of an aquarium. Therefore, it is recommended to rinse mechanical filters in an outside bucket of aquarium water to dislodge organic materials that contribute to nitrate problems, while preserving bacteria populations. Another safe practice consists of cleaning only one half of the filter media every time the filter or filters are serviced. | |
| + | ===Biological Loading=== <!--T:51--> | ||
| + | Biological loading is a measure of the burden placed on the aquarium ecosystem by its living inhabitants. High biological loading in an aquarium represents a more complicated tank ecology, which in turn means that equilibrium is easier to perturb. In addition, there are several fundamental constraints on biological loading based on the size of an aquarium. The surface area of water exposed to air limits dissolved oxygen intake by the tank. The capacity of nitrifying bacteria is limited by the physical space they have available to colonize. Physically, only a limited size and number of plants and animals can be fit into an aquarium while still providing room for movement. | ||
| + | ====Calculating aquarium capacity==== | ||
| + | An aquarium can only support a certain number of fish. Limiting factors include the availability of oxygen in the water and the rate at which the filter can process waste. Aquarists have developed a number of rules of thumb to allow them to estimate the number of fishes that can be kept in a given aquarium; the examples below are for small freshwater fish as larger freshwater fish and most marine fishes need much more generous allowances. | ||
| − | + | <!--T:52--> | |
| + | * 3 cm of fish length per 4 liters of water (i.e., a 6 cm-long fish would need about 8 liters of water). | ||
| + | * 1 cm of fish length per 30 square centimeters of surface area. | ||
| + | * 1 inch of fish length per gallon of water. | ||
| + | * 1 inch of fish length per 12 square inches of surface area. | ||
| − | + | <!--T:53--> | |
| + | Experienced aquarists warn against applying these rules too strictly because they do not consider other important issues such as growth rate, activity level, social behavior, and so on. To some degree, establishing the maximum loading capacity of an aquarium depends upon slowly adding fish and monitoring water quality over time, essentially a trial and error approach. | ||
| − | == | + | ====Factors affecting capacity==== <!--T:54--> |
| − | |||
| − | + | <!--T:55--> | |
| − | + | Though many conventional methods of calculating the capacity of aquarium is based on volume and pure length of fish, there are other variables. One variable is differences between fish. Smaller fish consume more oxygen per gram of body weight than larger fish. Labyrinth fish, having the capability to breathe atmospheric oxygen, are noted for not needing as much surface area (however, some of these fish are territorial, and may not appreciate crowding). Barbs also require more surface area than tetras of comparable size. | |
| − | + | <!--T:56--> | |
| + | Oxygen exchange at the surface is an important constraint, and thus the surface area of the aquarium. Some aquarists go so far as to say that a deeper aquarium with more volume holds no more fish than a shallower aquarium of the same surface area. The capacity can be improved by surface movement and water circulation such as through aeration, which not only improves oxygen exchange, but also the decomposition of waste materials. | ||
| − | The | + | <!--T:57--> |
| + | The presence of waste materials presents itself as a variable as well. Decomposition is an oxygen-consuming process, therefore the more decaying matter there is, the less oxygen as well. Oxygen dissolves less readily in warmer water; this is a double-edged sword as warmer temperatures make more active fish, which in turn consume even more oxygen. Stress due to temperature changes is especially obvious in coldwater aquaria where the temperature may swing from low temperatures to high temperatures on hotter days. | ||
| − | + | <!--T:97--> | |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 5 --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=6}} | ||
| + | <noinclude><translate><!--T:98--> | ||
| + | </noinclude> | ||
| + | <!-- 6. Fill an aquarium containing at least five gallons of water with a balance of plants and fishes, either tropical or native, and maintain the same for at least six months. --> | ||
| + | This can be done by the individual Pathfinder at home, or as a group in a classroom or the regular Pathfinder meeting place. If keeping fish as a group, be sure that everyone gets to participate in all aspects of their care. The likelihood of success will be maximized by being sure to follow the guidelines set out in requirement five. | ||
| − | = | + | <!--T:99--> |
| − | + | <noinclude></translate></noinclude> | |
| + | {{CloseReq}} <!-- 6 --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=7}} | ||
| + | <noinclude><translate><!--T:100--> | ||
| + | </noinclude> | ||
| + | <!-- 7. Note the effect of the following on the fishes and aquarium in general: --> | ||
| + | <noinclude></translate></noinclude> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=7a}} | ||
| + | <noinclude><translate><!--T:101--> | ||
| + | </noinclude> | ||
| + | During the time that aquarium plants are exposed to light, carbon dioxide is absorbed and oxygen is expelled. The gases enter the plant mainly through the leaves. The carbon dioxide and water are chemically combined with the chlorophyll in the plant to produce simple sugars. The sugars are converted to starch and oxygen is produced as the by-product. The light in your tank is most important with respect to the chlorophyll. The chlorophyll is what absorbs the light to create the process of photosynthesis. The aquarium plant naturally absorbs more nutrients through the roots during this time. | ||
| − | + | <!--T:102--> | |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 7a --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=7b}} <!--T:60--> | ||
| + | <noinclude><translate><!--T:103--> | ||
| + | </noinclude> | ||
| + | Respiration is the opposite of photosynthesis. When the lights are out, the photosynthesis process ceases but the respiration continues. The aquarium plant will use oxygen to break down food substances, which is released as energy in the form of heat. Carbon dioxide is produced and expelled as a result of this process. | ||
| + | So, when the lights are on the plants absorb carbon dioxide and expel oxygen. When the lights are out the aquarium plants absorb oxygen and expel carbon dioxide. | ||
| − | + | <!--T:104--> | |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 7b --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=7c}} <!--T:61--> | ||
| + | <noinclude><translate><!--T:105--> | ||
| + | </noinclude> | ||
| + | Overfeeding is one of the major causes of fish loss. Overfeeding promotes fish waste (ammonia) to build up to a harmful level. It is best to feed your betta only enough food that it can eat in five minutes. If food is seen sitting on the bottom of the aquarium or bowl, the fish have been overfed. | ||
| − | + | <!--T:106--> | |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 7c --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=7d}} <!--T:62--> | ||
| + | <noinclude><translate><!--T:107--> | ||
| + | </noinclude> | ||
| − | |||
| − | + | <!--T:63--> | |
| + | Rapid changes in water temperatures stress your fish. when fish are stressed they are more susceptible to disease and sickness. | ||
| − | + | <!--T:108--> | |
| − | <!-- -- | + | <noinclude></translate></noinclude> |
| − | + | {{CloseReq}} <!-- 7d --> | |
| − | + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=7e}} <!--T:64--> | |
| − | + | <noinclude><translate><!--T:109--> | |
| − | ------ | + | </noinclude> |
| − | < | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | <!--T:65--> | |
| − | + | Fish need plants in the wild for shelter, food, filtration, and oxygen. In an aquarium you supply their main source of food | |
| − | + | <!--T:110--> | |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 7e --> | ||
| + | {{CloseReq}} <!-- 7 --> | ||
| + | <noinclude><translate></noinclude> | ||
| + | ==Notes== <!--T:66--> | ||
| + | When to use ''fish'' or ''fishes''. Use ''fish'' when talking about a school of the same species. Use ''fishes'' when talking about schools of different species. Any time you are talking about more than one species, use ''fishes''. <ref>http://www.amonline.net.au/fishes/what/fish.htm</ref> | ||
| − | + | ==References== <!--T:67--> | |
| − | [[ | + | *[[w:List of freshwater aquarium fish species|List of freshwater aquarium fish species]] |
| − | + | <references/> | |
| − | + | <noinclude></translate></noinclude> | |
| − | + | {{CloseHonorPage}} | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Latest revision as of 18:19, 14 July 2022
1
2
- a. Explain their breeding habits.
- b. Give the habitat or country in which they are found.
Neon Tetra
Neon Tetra (Paracheirodon innesi)
Where found: The Neon Tetra is native to blackwater or clearwater streams in southeastern Colombia, eastern Peru, and western Brazil, including the tributaries of the Solimões. Fish are collected in warm-flowing (21–29°C) clear and blackwater streams, but never in whitewater rivers of Andean origin.
Description: The Neon Tetra has a dark olive-green back over a silver-white abdomen. The fish is characterized by an iridescent blue horizontal stripe along each side of the fish from its nose to the base of the adipose fin, and an iridescent red stripe that begins at the middle of the body and extends posteriorly to the base of the caudal fin. During the night, the color disappears as the fish rests—it reactivates once it becomes active in the morning. It grows to approximately 3 cm (1.25 in) in overall length. Sexual dimorphism is slight, the female having a slightly larger belly.
Reproduction: To breed Neon Tetras, place a pair of the species in a breeding tank without any light, and gradually increase the lighting until spawning occurs. Other inducers include mosquito larvae and a hardness of less than 4 degrees. Some also recommend letting the level of nitrates rise, then do at least 50% water change to simulate the fresh rain the tetras get in their natural habitat, the Amazon. It is recommended that everything you place in the aquarium be sterilized, as well as the aquarium top. Because the adults will often eat newly-hatched fry, it is best to remove them as soon as the eggs have been laid. The eggs are especially sensitive to light. Eggs will hatch within 24 hours of the laying. Fry can be fed rotifers, especially infusoria and egg yolk for 1 to 4 weeks, followed by nauplii of brine shrimp, shaved cattle liver, and formulated diets. Fry will achieve their adult coloration at approximately one month of age. Adults can spawn every two weeks.
Afra Cichlid
Afra Cichlid (Cynotilapia afra)
Where found: This fish is endemic to the central and northern parts of Lake Malawi and is found in rocky habitats.
Description: The afra cichlid has an elongate body typically with vertical blue and black bars. However, there are many different colorations depending on the region the fish is from, for example, the male fish from Kobwe have greenish stripes on blue-black body. They can grow up to 10 cm. Like many other cichlid from Lake Malawi, afra cichlids are mouthbrooders. Males defend boulders as their territories and feed from algae on those boulders. Females congregate in mid-water and feed from plankton.
Reproduction: Like many mbuna cichlids, this is a notoriously aggressive fish that should be kept in a species or mbuna tank. The best practice is to keep one male with several females. They should be provided with large spaces with plenty of hiding shelters.
Angel Fish
Angel Fish (Pterophyllum spp.)
Where found: All Pterophyllum species originate from the Amazon River basin in tropical South America.
Description: The three species of Pterophyllum are unusually shaped for cichlids being greatly laterally compressed, with round bodies and elongated triangular-shaped dorsal and anal fins. This body shape allows them to hide among roots and plants, often on a vertical surface. Naturally occurring angelfish are frequently striped longitudinally, colouration which provides additional camouflage. Angelfish are ambush predators and prey on small fish and macroinvertebrates. All Pterophyllum species form monogamous pairs. Eggs are generally laid on a submerged log or a flattened leaf. As is the case for other cichlids, brood care is highly developed.
Reproduction: P. scalare is relatively easy to breed in the aquarium, although one of the results of generations of inbreeding is that many breeds have almost completely lost their rearing instincts resulting in the tendency of the parents to eat their young. In addition, it is very difficult to accurately identify the gender of any individual until they are nearly ready to breed. Angelfish pairs form long-term relationships where each individual will protect the other from threats and potential suitors. Upon the death or removal of one of the mated pair, some breeders have experienced a total refusal of the other mate to pair up with any other angelfish; others have had more success with subsequent mates. Both parents care for the young. Depending upon aquarium conditions, P. scalare reaches sexual maturity at the age of six to twelve months or more. In situations where the eggs are removed from the aquarium immediately after spawning, the pair is capable of spawning every seven to ten days. Around the age of approximately three years, spawning frequency will decrease and eventually cease. When the pair is ready to spawn, they will choose an appropriate medium upon which to lay the eggs and spend one to two days picking off detritus and algae from the surface. This medium may be a broad-leaf plant in the aquarium, a flat surface such as a piece of slate placed vertically in the aquarium, a length of pipe, or even the glass sides of the aquarium. The female will deposit a line of eggs on the spawning substrate, followed by the male who will fertilize the eggs. This process will repeat itself until there are a total of 100 to up to 1200+ eggs, depending on the size and health of the female fish. The pair will take turns maintaining a high rate of water circulation around the eggs by swimming very close to the eggs and fanning the eggs with their pectoral fins. In a few days, the eggs hatch and the fry remain attached to the spawning substrate. During this period, the fry will not eat and will survive by consuming the remains of their yolk sacs. At one week, the fry will detach and become free-swimming. Successful parents will keep close watch on the eggs until they become free-swimming. At the free-swimming stage, the fry can be fed newly-hatched frozen och fresh (i.e. alive) brine shrimp (Artemia spp.).
Discus
Discus (Symphysodon spp.)
Where found: Discus (Symphysodon spp.) are a genus of three species of freshwater cichlid fishes native to the Amazon River basin
Description: Like cichlids from the genus Pterophyllum, all Symphysodon species have a laterally compressed body shape. In contrast to Pterophyllum, however, extended finnage is absent giving Symphysodon a more rounded shape. It is this body shape from which their common name, “discus”, is derived. The sides of the fish are frequently patterned in shades of green, red, brown, and blue. The height and length of the grown fish are both about 20–25 cm![]() .
.
Reproduction: Another characteristic of Symphysodon species is the way they care for their larvae. As for most cichlids, brood care is highly developed with both the parents caring for the young. Additionally, adult discus produce a secretion through their skin, off which the larvae live during their first few days. This behaviour has also been observed for Uaru species.
Gold Barb
Gold Barb (Puntius semifasciolatus)
Where found: Its native habitat is the Red River basin in southeast China.
Description: The gold barb is a medium-long barb. Adults have highly arched backs and a short pair of barbels on the upper jaw at the corners of the mouth. The back is light to reddish brown, the sides are metallic green or yellow-green, with a brassy or golden sheen below. The belly is whitish, turning orange-red in males at mating time. Females can be distinguished by their dull colors and their overall bulk. The average size of adults is 7 - 8 cm![]() .
.
Reproduction: An egg-scatterer, adult barbs will spawn around a hundred eggs. This breeding occurs at the first light in the early morning.
Guppy
Guppy (Poecilia reticulata)
Where found: Guppies are native to Trinidad and parts of South America, specifically Antigua and Barbuda, Barbados, Brazil, Guyana, Netherlands Antilles, Trinidad and Tobago, the US Virgin Islands, and Venezuela.
Description: The guppy (Poecilia reticulata) is one of the most popular freshwater aquarium fish species in the world. It is a small member of the Poecilidae family (females 4-6 centimeters long, males 2½–3½ centimeters long) and like all other members of the family, is live-bearing.
Reproduction: Guppies are highly prolific livebearers. The gestation period of a guppy is 22-30 days, with an average of 28 days. After the female guppy is inseminated, a dark area near the anus, known as the gravid spot, will enlarge and darken. Guppies prefer water temperatures of about 28 °C![]() for reproduction. The female guppy drops of between 2-100 fry, typically ranging between 30 and 60. After giving birth, the female is ready for conception again within only a few hours.
for reproduction. The female guppy drops of between 2-100 fry, typically ranging between 30 and 60. After giving birth, the female is ready for conception again within only a few hours.
Molly
Molly (Poecilia sphenops)
Where found: Mollies inhabit the coastal brackish and marine waters of Mexico.
Description: This species is one of the most well-known aquarium fishes and nearly as easy to keep and prolific as guppies (for optimal health and breeding success, they demand fresh vegetable food like algae). There are several other popular breeds, like the golden molly nicknamed "24 karat", or the balloon molly, which however has a deformed spine and a decreased lifespan due to the associated health problems. Also, breeds with altered caudal fin structures like lyretails exist. The wild form is in fact quite rarely kept, as it has a rather plain silvery coloration suffused with brown and green hues. If given good care with ample sunlight, high water temperatures and fresh vegetables, they will, however, prove charming fish who make up for their somewhat plain coloration with their lively behavior.
Reproduction: Fertilization is internal and is accomplished by means of highly modified fin elements within the anal fin of males that form a structure known as the gonopodium. Sailfin mollies produce broods of 10-140 live young, depending upon maturity and size, and females may store sperm long after the demise of their relatively short-lived mates. The gestation period for this species is approximately 3-4 weeks, depending upon temperature, and a single female may give birth on multiple occasions throughout the year. Although sex ratios of the broods are balanced, adult populations tend to be largely female as males appear to suffer higher rates of mortality due to a greater susceptibility to predators and disease as a consequence of their brighter colours and a life devoted to frenzied breeding. There is no parental care exhibited by this species.
Betta
Betta (Betta)
Where found: The Siamese fighting fish (Betta splendens) is one of the most popular species of freshwater aquarium fish. It is native to the Mekong river basin in Southeast Asia.
Description: Betta Bleeker, 1850 is a large genus of small, often colorful, freshwater ray-finned fishes. All the Betta species are small fishes, but they vary considerably in size, ranging from under 2.5 cm (1 inch) total length in B. chanoides to 14 cm (5.5 inches) in the Akar betta (B. akarensis).[1] Bettas are anabantoids, which means they can breathe atmospheric air thanks to a unique organ called the labyrinth. This accounts for their ability to thrive in low-oxygen water conditions that would kill most other fish, such as rice paddies, slow-moving streams, drainage ditches, and large puddles.
Reproduction: The various bettas can be divided into two groups, based on their spawning behavior: some build bubble nests, like B. splendens, while others are mouthbrooders, like B. picta. The mouthbrooding species are sometimes called "pseudo bettas".
Goby
Goby (Elacatinus)
Where found: Neon gobies are native to the tropical reefs of the Gulf of Mexico, from Texas to Belize, where they live primarily in the rocks.
Description: Neon gobies are very small, torpedo-shaped fish. Although sizes vary slightly by species, they are generally about 2.5 cm![]() long. They have dark bodies with iridescent stripes (the color of which varies by species) running from the tip of the nose to the base of the caudal fin. Like all gobies, their dorsal fin is split in two, the anterior dorsal fin being rounded like that of a clownfish and the posterior dorsal fin being relatively flat. The anal fin lines up with the posterior dorsal fin and is of similar shape. The pectoral fins are nearly circular, and, like all other fins, transparent.
They are well-documented cleaner fish, setting up stations where often much larger fish (sometimes even fish who would normally eat the gobies) come to have the gobies eat their small external parasites. This is an excellent example of symbiosis – the cleaned fish are healthier and the gobies have not only an excellent food source but also relative protection from potential predators.
long. They have dark bodies with iridescent stripes (the color of which varies by species) running from the tip of the nose to the base of the caudal fin. Like all gobies, their dorsal fin is split in two, the anterior dorsal fin being rounded like that of a clownfish and the posterior dorsal fin being relatively flat. The anal fin lines up with the posterior dorsal fin and is of similar shape. The pectoral fins are nearly circular, and, like all other fins, transparent.
They are well-documented cleaner fish, setting up stations where often much larger fish (sometimes even fish who would normally eat the gobies) come to have the gobies eat their small external parasites. This is an excellent example of symbiosis – the cleaned fish are healthier and the gobies have not only an excellent food source but also relative protection from potential predators.
Reproduction: If kept in pristine conditions and fed well neon gobies will readily spawn in home aquaria. A species or breeding tank is required, as the fry are small and will be eaten by most other fish. The gobies are sexually dimorphic, but the difference is not easy to ascertain so they are normally kept in large groups to ensure a balance of sexes. They will lay their eggs on any hard surface along the bottom, and the fry, which feed on small rotifers or other microscopic organisms, are fully developed within a month. The average lifespan for a neon goby is approximately a year to a year and a half.
Green Swordtail
Xiphophorus hellerii
Green Swordtail (Xiphophorus hellerii)
Where found: It is native to an area of North and Central America stretching from Veracruz, Mexico, to northwestern Honduras.
Description: The male green swordtail grows to a maximum overall length of 14cm![]() and the female to 16 cm
and the female to 16 cm![]() . The name "swordtail" is commonly but mistakenly believed to be derived form the elongated lower lobe of the male's caudal fin (tailfin), but is actually derived from the sword shaped anal fin of the male. Sexual dimorphism is moderate, with the female being larger than the male but lacking the "sword". The wild form is olive green in color, with a red or brown lateral stripe and speckles on the dorsal and, sometimes, caudal fins. The male's "sword" is yellow, edged in black below. Captive breeding has produced many color varieties, including black, red, and many patterns thereof, for the aquarium hobby.
. The name "swordtail" is commonly but mistakenly believed to be derived form the elongated lower lobe of the male's caudal fin (tailfin), but is actually derived from the sword shaped anal fin of the male. Sexual dimorphism is moderate, with the female being larger than the male but lacking the "sword". The wild form is olive green in color, with a red or brown lateral stripe and speckles on the dorsal and, sometimes, caudal fins. The male's "sword" is yellow, edged in black below. Captive breeding has produced many color varieties, including black, red, and many patterns thereof, for the aquarium hobby.
Reproduction: The males' elongated caudal fins have been found to significantly affect their chances at mating. The presence of a well-endowed male spurs the maturity of females while it inhibits the maturity of juvenile males in the vicinity as the well-endowed male.
3
The fish presented here are native to the United States and Canada.
Brook Trout
Brook Trout (Salvelinus fontinalis)
Where found: The brook trout is native to small streams, creeks, lakes, and spring ponds. Some brook trout are anadromous. It is native to a wide area of eastern North America but increasingly confined to higher elevations southward in the Appalachian Mountains to northern Georgia, Canada from the Hudson Bay basin east, the Great Lakes–Saint Lawrence system, and the upper Mississippi River drainage as far west as eastern Iowa.
Description: The brook trout is of dark green to brown basic coloration with a distinctive marbled pattern (called vermiculations) of lighter shades across the flanks and back and extending at least to the dorsal fin, and often to the tail. There is a distinctive sprinkling of red dots, surrounded by blue haloes, along the flank. The belly and lower fins are reddish in color, the latter with white leading edges. Often the belly, particularly of the males, becomes very red or orange when the fish are spawning. The species reaches a maximum recorded length of 86 cm (33 in) and a maximum recorded weight of 9.4 kg (21 lb). It can reach at least seven years of age, with reports of 15-year-old specimens observed in California habitats to which the species has been introduced.
Diet: Its diverse diet includes crustaceans, frogs and other amphibians, insects, molluscs, smaller fish, and even small aquatic mammals such as voles.
Reproduction: Individuals normally spend their entire life in fresh water, but some — colloquially called "salters" or "sea run" — may spend up to three months at sea in the spring, not straying more than a few kilometers from the river mouth. The fish return upstream to spawn in the late summer or autumn. The female constructs a depression in a location in the stream bed, sometimes referred to as a "redd", where groundwater percolates upward through the gravel. One or more males approaches the female, fertilizing the eggs as the female expresses them. The eggs are slightly more dense than water. The female then buries the eggs in a small gravel mound. The eggs hatch in approximately 100 days.
Rainbow Trout
Rainbow Trout (Oncorhynchus mykiss)
Where found: The rainbow trout is a species of salmonid native to tributaries of the Pacific Ocean in Asia and North America as well as much of the central, western, eastern, and especially the northern portions of the United States.
Description: The rainbow trout are unusual in that there are two forms which sometimes share the same habitat. The anadromous (sea-going) form called "steelhead" migrate to the ocean, though they must return to fresh water to reproduce. The freshwater form is called "rainbow trout", based on the broad red band along their sides. Steelhead are exactly the same species as rainbow trout. However, the difference is anadromy. After going to sea, their color changes, including loss of the red band. They stay at sea for 1-4 years, and return to fresh water to spawn. Rainbows stay in fresh water their whole lives. Rainbows and steelhead occur in well-oxygenated lakes and streams where the temperature normally doesn't rise above 12°C in summer. Rainbows range from 12 to 36 inches in length. Steelhead grow longer, ranging from 50 to 122 cm (20 to 48 inches) in length. Steelhead range in weight from 2.5 kg to 10 kg (5.5 - 22 pounds).
Diet: Rainbow trout have a varied diet. They are predators, eating any smaller fish from nearly the time they are born. Insects make up a large portion of the diet, along with crayfish and other crustaceans, some lake dwelling species may become planktonic feeders. Trout of all ages will eat nearly anything they can grab, in contrast with the legendary, selective nature the fish often gets. They are near the top of the food chain in most freshwater environments. However they are lower on the rung of other freshwater predators such as pike, muskie, lake trout, and chinook salmon. Rainbows will take fish up to and over 1/3 of their length and larger.
Reproduction: Unlike other Pacific Salmon, rainbow trout and steelhead do not necessarily die after spawning (they may spawn as many as four times).
Northern Pike
Northern Pike (Esox lucius)
Where found: E. lucius is found throughout the northern hemisphere, including Russia, Europe, the British Isles, and North America.
Description: Northern pike are most often olive, shading into yellow to white along the belly. The flank is marked with short, light barlike spots and there are a few to many dark spots on the fins. The lower half of the gill cover lacks scales and they have large sensory pores on their head and on the underside of the lower jaw which are part of the lateral line system. Unlike the similar-looking and closely related muskellunge, the northern pike has light markings on a dark body background and fewer than six sensory pores on the underside of each side of the lower jaw.
Diet: Pike are typical ambush predators; they lie in wait for prey, holding perfectly still for long periods and then exhibit remarkable acceleration as they strike. The fish has a distinctive habit of catching its prey sideways in the mouth, killing or immobilizing it with its sharp teeth, and then turning the prey lengthwise to swallow it. It eats mainly fish, but on occasion water voles and ducklings have also been known to fall prey to pike. Pike will aggressively strike at any fish in the vicinity, even at other pike. Young pike have been found dead from choking on a pike of a similar size. Northern pike also feed on frogs, insects and leeches. It has often been suggested that pike optimally forage on prey that are from 25 to 35% of their body length. Also on rare occasions pike have been reported to have eaten young bald eagles.
Reproduction: The Northern Pike spawns beneath the ice in the Spring. A female is followed by four or five smaller males as she seeks vegetative cover in the shallows. When the eggs are released and fertilized, the stick to the vegetation where they are abandoned by the parents.
Bluegill
Bluegill (Lepomis macrochirus)
Where found: The bluegill is native to a wide area of North America, from Québec to northern Mexico, and has been widely transplanted to stock game fish for anglers.
Description: The Bluegill is a species of freshwater fish sometimes referred to as bream, brim, or copper nose. The bluegill's most notable feature is the blue or black "ear", actually an extension of the gill cover called the opercular flap. Its name, however, comes from the bright blue edging visible on its gill rakers. It can be distinguished from similar species by the (not always pronounced) vertical bars along its flanks. The bluegill grows to a maximum overall length of approximately 40 cm (16 in).
Diet: The natural diet of the bluegill consists largely of small invertebrates and very small fish.
Reproduction: These fish spawn in June in nests in the shallows. During this period males assume a very bold coloration, as they are guarding their nests. An interesting aspect of their biology is that some males assume the coloration of the female fish so that the nest-guarding males won't show aggression towards them. Then these "sneaker" males enter nests and spawn.
Smallmouth Bass
Smallmouth Bass (Micropterus dolomieu)
Where found: The smallmouth bass is native to the upper and middle Mississippi River basin, the Saint Lawrence River–Great Lakes system, and up into the Hudson Bay basin.
Description: The smallmouth bass is generally green with dark vertical bands rather than a horizontal band along the side. There are 13-15 soft rays in the dorsal fin. The upper jaw of smallmouth bass does not extend beyond the back of the eye.
Diet: Carnivorous, its diet comprises crayfish, insects, and smaller fish, the young also feeding on zooplankton.
Reproduction: The female can lay up to 21,000,000 eggs, which are guarded by the male in his nest.
Largemouth Bass
Micropterus salmoides
Largemouth Bass (Micropterus salmoides)
Where found: Native to the central and southeastern United States, the largemouth bass has been introduced throughout most of the United States and Canada.&
Description: The largemouth is marked by a series of dark, sometimes black, blotches forming a jagged horizontal stripe along each flank. The upper jaw of a largemouth bass extends beyond the rear edge of the eye.
Diet: The juvenile largemouth bass consumes mostly zooplankton and insects. Adults consume small fish, crayfish, and frogs. Prey items can be as large as 25 to 35% of the bass's body length. Largemouth bass have even been reported to take small birds, small mammals, such as mice and rats and small snakes.
Reproduction: Spawning occurs in shallow areas of lakes and ponds in the spring. Males arrive first, selecting a territory on fine gravel, coarse sand or among sparse vegetation. The male will slightly clear, using his fins, a shallow depression into which he will entice a female to deposit her eggs. Females can lay up to a million eggs during each season. The male guards the embryos until the larvae hatch and then will continue to guard the "fry" until they disperse from the nest. During the guarding period, the male ferociously attacks any potential predators that approach too closely.
White Perch
White Perch (Morone americana)
Where found: Although favoring brackish waters, it is also found in fresh water and coastal areas from the St. Lawrence River and Lake Ontario south to the Pee Dee River in South Carolina.
Description: Generally silvery-white in color, hence the name, it has been reported up to 49.5 cm in length and weighing 2.2 kg.
Diet: White perch are known to eat the eggs of many species native to the Great Lakes, such as walleye and other true perches. At times, fish eggs are 100% of its diet.
Reproduction: White perch are a prolific species. The female can deposit over 140,000 eggs in a spawning session, lasting just over a week. Several males will often attend a spawning female, and each may fertilize a portion of her eggs. The young hatch within 1 to 6 days of fertilization.
Black Crappie
Black Crappie (Pomoxis nigromaculatus)
Where found: The crappie is native throughout the eastern half of Canada and the United States, and has been widely introduced in the west as well. As of 2005, populations existed in all of the lower 48 states of the United States.
Description: The black crappue is most accurately identified by the seven or eight spines on its dorsal fin.
Diet: Adult crappies feed predominantly on smaller species, including the young of their own predators (which include the northern pike, muskellunge, and walleye). They have diverse diets, however, including zooplankton, insects, and crustaceans
Reproduction: The breeding season varies by location, due to the species’ great range; breeding temperature is 14‒20 °C (58‒68 °F) and spawning occurs between April and June. Spawning occurs in a nest built by the male, who guards the eggs and young.
Striped Bass
Striped Bass (Morone saxatilis)
Where found: Striped bass are found along the Atlantic coastline of North America from the St. Lawrence River into the Gulf of Mexico to approximately Louisiana. They are anadromous fish that migrate between fresh and salt water. Spawning takes place in freshwater.
Description: The striped bass is a typical member of the Moronidae family in shape, having a streamlined, silvery body marked with longitudinal dark stripes running from behind the gills to the base of the tail. Maximum size is 200 cm (6.6 ft) and maximum scientifically recorded weight 57 kg (125 US pounds). Striped bass are believed to live for up to 30 years.
Diet: Striped bass are primarily nocturnal feeders, eating insects, crustaceans, and other fish.
Reproduction: Striped bass spawn in freshwater and spend their adult lives in saltwater (i.e., it is anadromous). They can also live exclusively in freshwater and currently flourish in several inland water bodies.
Haddock
Haddock (Melanogrammus aeglefinus)
Where found: The haddock or offshore hake is a marine fish distributed on both sides of the North Atlantic.
Description: The haddock is easily recognized by a black lateral line running along its white side, not to be confused with pollock which has the reverse, ie white line on black side, and a distinctive dark blotch above the pectoral fin. They grow up to 1.1 meters![]() in length.
in length.
Diet: Haddock feed primarily on small invertebrates, although larger members of the species may occasionally consume fish.
Reproduction: Spawning occurs between January and June, peaking during late March and early April. The most important spawning grounds are in the waters off middle Norway near southwest Iceland, and Georges Bank. An average-sized female produces approximately 850,000 eggs, and larger females are capable of producing up to 3 million eggs each year.
4
4a
A dorsal fin is a fin located on the backs of fishes, whales, dolphins and porpoises, as well as the (extinct) ichthyosaurs. Its main purpose is to stabilize the animal against rolling and assist in sudden turns. Some animals have developed dorsal fins with protective functions, such as spines or venom. Many catfish can lock the leading ray of the dorsal fin in an extended position to discourage predation or to wedge themselves into a crevice.
Dorsal fins come in a variety of shapes and sizes.
4b
The paired pectoral fins are located on each side, usually just behind the operculum, and are homologous to the forelimbs of tetrapods. A peculiar function of pectoral fins, highly developed in some fish, is the creation of the dynamic lifting force that assists, e.g., sharks, in maintaining depths and enables the flight for flying fish.
4c
The paired pelvic or ventral fins are located ventrally below the pectoral fins. They are homologous to the hindlimbs of tetrapods.
4d
The anal fin is located on the ventral surface behind the anus. This fin is used to stabilize the fish while swimming.
4e
The caudal fin is the tail fin, located at the end of the caudal peduncle.
4f
The lateral line is a sense organ used to detect movement and vibration in the surrounding water. It consists of a line of receptors running along each side of the fish.
4g
The operculum of a bony fish is the hard bony flap covering and protecting the gills. In most fish, the rear edge of the operculum roughly marks the division between the head and the body. The operculum is composed of four bones; the opercle, preopercle, interopercle, and subopercle. The morphology of this anatomical feature varies greatly between species. For example, the bluegill (Lepomis macrochirus) has a posteriorly and dorsally oriented rounded extension with a small black splotch present. In some species, the operculum can push water from the buccal cavity through the gills.
For some fish, the operculum is vital in obtaining oxygen. It opens as the mouth closes, causing the pressure inside the fish to drop. Water then flows towards the lower pressure across the fish's gill lamellae, allowing some oxygen to be absorbed from the water.
Cartilaginous fishes do not have an operculum. Without an operculum, other methods of getting water to the gills are required, such as ventilation.
4h
The head may have several fleshy structures known as barbels, which may be very long and resemble whiskers.
4i
The gas bladder, or swim bladder, is an internal organ that contributes to the ability of a fish to control its buoyancy, and thus to stay at the current water depth, ascend, or descend without having to waste energy in swimming. It is often absent in fast swimming fishes such as the Tuna and Mackerel families.
4j
The gills, located under the operculum, are a respiratory organ for the extraction of oxygen from water and for the excretion of carbon dioxide. They are not usually visible, but can be seen in some species e.g. the frilled shark.
5
- a. Tropical zone
- b. Temperate zone
Ideal aquarium ecology reproduces the balance found in nature in the closed system of an aquarium. In practice it is virtually impossible to maintain a perfect balance. As an example, a balanced predator-prey relationship is nearly impossible to maintain in even the largest of aquaria. Typically, an aquarium keeper must take steps to maintain balance in the small ecosystem contained in his aquarium.
Approximate balance is facilitated by large volumes of water. Any event that perturbs the system pushes an aquarium away from equilibrium; the more water that is contained in a tank, the easier such a systemic shock is to absorb, as the effects of that event are diluted. For example, the death of the only fish in a three U.S. gallon tank (11 L) causes dramatic changes in the system, while the death of that same fish in a 100 U.S. gallon (400 L) tank with many other fish in it represents only a minor change in the balance of the tank. For this reason, hobbyists often favor larger tanks when possible, as they are more stable systems requiring less intensive attention to the maintenance of equilibrium.
There are a variety of nutrient cycles that are important in the aquarium. Dissolved oxygen enters the system at the surface water-air interface or through the actions of an air pump. Carbon dioxide escapes the system into the air. The phosphate cycle is an important, although often overlooked, nutrient cycle. Sulfur, iron, and micronutrients also cycle through the system, entering as food and exiting as waste. Appropriate handling of the nitrogen cycle, along with supplying an adequately balanced food supply and considered biological loading, is usually enough to keep these other nutrient cycles in approximate equilibrium. ===Water conditions===The solute content of water is perhaps the most important aspect of water conditions, as total dissolved solids and other constituents can dramatically impact basic water chemistry, and therefore how organisms are able to interact with their environment. Salt content, or salinity, is the most basic classification of water conditions. An aquarium may have freshwater (salinity below 0.5 PPT), simulating a lake or river environment; brackish water (a salt level of 0.5 to 30 PPT), simulating environments lying between fresh and salt, such as estuaries; and salt water or seawater (a salt level of 30 to 40 PPT), simulating an ocean or sea environment. Rarely, even higher salt concentrations are maintained in specialized tanks for raising brine organisms.
Several other water characteristics result from dissolved contents of the water, and are important to the proper simulation of natural environments. The pH of the water is a measure of the degree to which it is alkaline or acidic. Saltwater is typically alkaline, while the pH of fresh water varies more. Hardness measures overall dissolved mineral content; hard or soft water may be preferred. Hard water is usually alkaline, while soft water is usually neutral to acidic. Dissolved organic content and dissolved gases content are also important factors.
Home aquarists typically use modified tap water supplied through their local water supply network to fill their tanks. Because of the chlorine used to disinfect drinking water supplies for human consumption, straight tap water cannot be used. In the past, it was possible to "condition" the water by simply letting the water stand for a day or two, which allows the chlorine time to dissipate. However, chloramine is now used more often as it is much stabler and will not leave the water as readily. Additives formulated to remove chlorine or chloramine are often all that is needed to make the water ready for aquarium use. Brackish or saltwater aquaria require the addition of a mixture of salts and other minerals, which are commercially available for this purpose.
More sophisticated aquarists may make other modifications to their base water source to modify the water's alkalinity, hardness, or dissolved content of organics and gases, before adding it to their aquaria. This can be accomplished by a range of different additives, such as sodium bicarbonate to raise pH. Some aquarists will even filter or purify their water prior to adding it to their aquarium. There are two processes used for that: deionization or reverse osmosis. In contrast, public aquaria with large water needs often locate themselves near a natural water source (such as a river, lake, or ocean) in order to have easy access to a large volume of water that does not require much further treatment.
The temperature of the water forms the basis of one of the two most basic aquarium classifications: tropical vs. cold water. Most fish and plant species tolerate only a limited range of water temperatures: Tropical or warm water aquaria, with an average temperature of about 25 °C (77 °F), are much more common, and tropical fish are among the most popular aquarium denizens. Cold water aquaria are those with temperatures below what would be considered tropical; a variety of fish are better suited to this cooler environment. More importantly than the temperature range itself is the consistency in temperature; most organisms are not accustomed to sudden changes in temperatures, which could cause shock and lead to disease. Water temperature can be regulated with a combined thermometer and heater unit (or, more rarely, with a cooling unit).
Water movement can also be important in accurately simulating a natural ecosystem. Aquarists may prefer anything from still water up to swift simulated currents in an aquarium, depending on the conditions best suited for the aquarium's inhabitants. Water movement can be controlled through the use of aeration from air pumps, powerheads, and careful design of internal water flow (such as location of filtration system points of inflow and outflow).
Nitrogen cycle
Of primary concern to the aquarist is management of the biological waste produced by an aquarium's inhabitants. Fish, invertebrates, fungi, and some bacteria excrete nitrogen waste in the form of ammonia (which will convert to ammonium, in acidic water) and must then pass through the nitrogen cycle. Ammonia is also produced through the decomposition of plant and animal matter, including fecal matter and other detritus. Nitrogen waste products become toxic to fish and other aquarium inhabitants at high concentrations.
The Process
A well-balanced tank contains organisms that are able to metabolize the waste products of other aquarium residents. The nitrogen waste produced in a tank is metabolized in aquaria by a type of bacteria known as nitrifiers (genus Nitrosomonas). Nitrifying bacteria capture ammonia from the water and metabolize it to produce nitrite. Nitrite is also highly toxic to fish in high concentrations. Another type of bacteria, genus Nitrospira, converts nitrite into nitrate, a less toxic substance to aquarium inhabitants. (Nitrobacter bacteria were previously believed to fill this role, and continue to be found in commercially available products sold as kits to "jump start" the nitrogen cycle in an aquarium. While biologically they could theoretically fill the same niche as Nitrospira, it has recently been found that Nitrobacter are not present in detectable levels in established aquaria, while Nitrospira are plentiful.) This process is known in the aquarium hobby as the nitrogen cycle.
In addition to bacteria, aquatic plants also eliminate nitrogen waste by metabolizing ammonia and nitrate. When plants metabolize nitrogen compounds, they remove nitrogen from the water by using it to build biomass. However, this is only temporary, as the plants release nitrogen back into the water when older leaves die off and decompose.
Maintaining the Tank
Although informally called the nitrogen cycle by hobbyists, it is in fact only a portion of a true cycle: nitrogen must be added to the system (usually through food provided to the tank inhabitants), and nitrates accumulate in the water at the end of the process, or become bound in the biomass of plants. This accumulation of nitrates in home aquaria requires the aquarium keeper to remove water that is high in nitrates, or remove plants which have grown from the nitrates.
Aquaria kept by hobbyists often do not have the requisite populations of bacteria needed to detoxify nitrogen waste from tank inhabitants. This problem is most often addressed through two filtration solutions: Activated carbon filters absorb nitrogen compounds and other toxins from the water, while biological filters provide a medium specially designed for colonization by the desired nitrifying bacteria. Activated carbon and other substances, such as ammonia absorbing resins, will stop working when their pores get full, so these components have to be replaced with fresh stocks constantly.
New aquaria often have problems associated with the nitrogen cycle due to insufficient number of beneficial bacteria, known as the "New Tank Syndrome". Therefore, new tanks have to be "matured" before stocking them with fish. There are three basic approaches to this: the fishless cycle, the silent cycle, and slow growth.
No fish are kept in a tank undergoing a fishless cycle. Instead, small amounts of ammonia are added to the tank to feed the bacteria being cultured. During this process, ammonia, nitrite, and nitrate levels are tested to monitor progress. The silent cycle is basically nothing more than densely stocking the aquarium with fast-growing aquatic plants and relying on them to consume the nitrogen, allowing the necessary bacterial populations time to develop. According to anecdotal reports of aquarists specializing in planted tanks, the plants can consume nitrogenous waste so efficiently that the spikes in ammonia and nitrite levels normally seen in more traditional cycling methods are greatly reduced, if they are detectable at all. More commonly slow growth entails slowly increasing the population of fish over a period of 6 to 8 weeks, giving bacteria colonies time to grow and stabilize with the increase in fish waste.
The largest bacterial populations are found in the filter; efficient filtration is vital. Sometimes, a vigorous cleaning of the filter is enough to seriously disturb the biological balance of an aquarium. Therefore, it is recommended to rinse mechanical filters in an outside bucket of aquarium water to dislodge organic materials that contribute to nitrate problems, while preserving bacteria populations. Another safe practice consists of cleaning only one half of the filter media every time the filter or filters are serviced.
Biological Loading
Biological loading is a measure of the burden placed on the aquarium ecosystem by its living inhabitants. High biological loading in an aquarium represents a more complicated tank ecology, which in turn means that equilibrium is easier to perturb. In addition, there are several fundamental constraints on biological loading based on the size of an aquarium. The surface area of water exposed to air limits dissolved oxygen intake by the tank. The capacity of nitrifying bacteria is limited by the physical space they have available to colonize. Physically, only a limited size and number of plants and animals can be fit into an aquarium while still providing room for movement.
Calculating aquarium capacity
An aquarium can only support a certain number of fish. Limiting factors include the availability of oxygen in the water and the rate at which the filter can process waste. Aquarists have developed a number of rules of thumb to allow them to estimate the number of fishes that can be kept in a given aquarium; the examples below are for small freshwater fish as larger freshwater fish and most marine fishes need much more generous allowances.
- 3 cm of fish length per 4 liters of water (i.e., a 6 cm-long fish would need about 8 liters of water).
- 1 cm of fish length per 30 square centimeters of surface area.
- 1 inch of fish length per gallon of water.
- 1 inch of fish length per 12 square inches of surface area.
Experienced aquarists warn against applying these rules too strictly because they do not consider other important issues such as growth rate, activity level, social behavior, and so on. To some degree, establishing the maximum loading capacity of an aquarium depends upon slowly adding fish and monitoring water quality over time, essentially a trial and error approach.
Factors affecting capacity
Though many conventional methods of calculating the capacity of aquarium is based on volume and pure length of fish, there are other variables. One variable is differences between fish. Smaller fish consume more oxygen per gram of body weight than larger fish. Labyrinth fish, having the capability to breathe atmospheric oxygen, are noted for not needing as much surface area (however, some of these fish are territorial, and may not appreciate crowding). Barbs also require more surface area than tetras of comparable size.
Oxygen exchange at the surface is an important constraint, and thus the surface area of the aquarium. Some aquarists go so far as to say that a deeper aquarium with more volume holds no more fish than a shallower aquarium of the same surface area. The capacity can be improved by surface movement and water circulation such as through aeration, which not only improves oxygen exchange, but also the decomposition of waste materials.
The presence of waste materials presents itself as a variable as well. Decomposition is an oxygen-consuming process, therefore the more decaying matter there is, the less oxygen as well. Oxygen dissolves less readily in warmer water; this is a double-edged sword as warmer temperatures make more active fish, which in turn consume even more oxygen. Stress due to temperature changes is especially obvious in coldwater aquaria where the temperature may swing from low temperatures to high temperatures on hotter days.
6
This can be done by the individual Pathfinder at home, or as a group in a classroom or the regular Pathfinder meeting place. If keeping fish as a group, be sure that everyone gets to participate in all aspects of their care. The likelihood of success will be maximized by being sure to follow the guidelines set out in requirement five.
7
7a
During the time that aquarium plants are exposed to light, carbon dioxide is absorbed and oxygen is expelled. The gases enter the plant mainly through the leaves. The carbon dioxide and water are chemically combined with the chlorophyll in the plant to produce simple sugars. The sugars are converted to starch and oxygen is produced as the by-product. The light in your tank is most important with respect to the chlorophyll. The chlorophyll is what absorbs the light to create the process of photosynthesis. The aquarium plant naturally absorbs more nutrients through the roots during this time.
7b
Respiration is the opposite of photosynthesis. When the lights are out, the photosynthesis process ceases but the respiration continues. The aquarium plant will use oxygen to break down food substances, which is released as energy in the form of heat. Carbon dioxide is produced and expelled as a result of this process.
So, when the lights are on the plants absorb carbon dioxide and expel oxygen. When the lights are out the aquarium plants absorb oxygen and expel carbon dioxide.
7c
Overfeeding is one of the major causes of fish loss. Overfeeding promotes fish waste (ammonia) to build up to a harmful level. It is best to feed your betta only enough food that it can eat in five minutes. If food is seen sitting on the bottom of the aquarium or bowl, the fish have been overfed.
7d
Rapid changes in water temperatures stress your fish. when fish are stressed they are more susceptible to disease and sickness.
7e
Fish need plants in the wild for shelter, food, filtration, and oxygen. In an aquarium you supply their main source of food
Notes
When to use fish or fishes. Use fish when talking about a school of the same species. Use fishes when talking about schools of different species. Any time you are talking about more than one species, use fishes. &