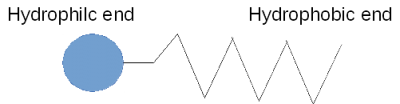Respuestas para la especialidad JA de Burbujas
Nivel de destreza
2
Año
2015
Version
08.02.2026
Autoridad de aprobación
División Norteamericana
1
1a
1b
1c
1d
1e
1f
2
2a
2b
2c
2d
3
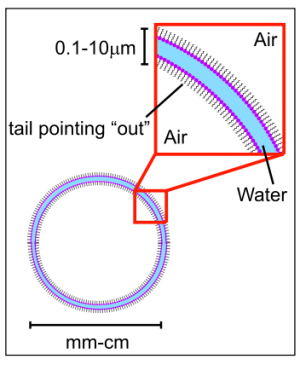
A soap molecule has a hydrophobic end and a hydrophilic end. In other words, it is both hydrophilic and hydrophobic, and because of this, it corrals water, separating it from the air as shown here.
4
- Soap solution will sting your eyes- avoid getting it in your eyes.
- Soap Solution is very Slippery- areas might still be slippery long after you are gone, so clean up any spills
- Never make bubbles near moving traffic.
- Soap solution can damage grass- dilute with water when finished.
- Don’t drink the bubble solution
5
5a
5b
- Procedure
6
6a
6b
7
7a
7b
7c
7d
Glycerin This is the secret ingredient that gives a bubble its extra strength.
7e
8
8a
8b
8c
Soap bubbles will assume the shape of least surface area possible containing a given volume. No matter what shape a bubble has initially, it will try to become a sphere. The sphere is the shape that minimizes the surface area of the structure, which makes it the shape that requires minimum energy to achieve.
Experiment
Bend pipe cleaners into several different shapes. Make as many different shapes as you can. You might want to make some square, some circles, some squares, some triangles, some heart-shaped or some even the shapes of letters. Make sure each one has a handle.
Carefully pour the bubble solution in a pie pan. Take each shaped pipe cleaner and dip it into the solution. Now start your bubble blowing.
What shapes are the Bubbles?
Record your Data.