Difference between revisions of "AY Honors/Water Science - Advanced/Answer Key"
(Marked this version for translation) |
|||
| (16 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | < | + | {{HonorSubpage}} |
| − | <noinclude><translate><!--T: | + | <section begin="Body" /> |
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=1}} | ||
| + | <noinclude><translate><!--T:34--> | ||
</noinclude> | </noinclude> | ||
| − | {{honor | + | <!-- 1. Have the Water Science honor. --> |
| − | + | {{honor_prerequisite|honor=Water Science}} | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | }} | ||
| − | + | <!--T:35--> | |
| − | {{ | + | <noinclude></translate></noinclude> |
| + | {{CloseReq}} <!-- 1 --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=2}} | ||
| + | <noinclude><translate><!--T:36--> | ||
| + | </noinclude> | ||
| + | <!-- 2. Illustrate and briefly describe how does each physical state of water contribute to the earth’s climate. --> | ||
| + | ;Solid (ice): reflects the sun’s heat, thus keeping the earth cool by limiting the amount of light that is turned into heat. Ice also helps to control the temperature of the earth by being a sink for heat. | ||
| + | ;Liquid (water): has a natural ability to absorb carbon dioxide from the atmosphere. This process allows the temperature of the earth to come down. As the sun shines on water, the absorbed light increases the temperature of the water. This process of heating and cooling allows for water to maintain a stable temperature. | ||
| + | ;Gas (vapor): plays a critical role in maintain earth’s weather. The process of water evaporation allows for the formation of clouds. As the clouds become heavy, they produce rain, which cleans the air and waters vegetation. | ||
| − | = | + | <!--T:37--> |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 2 --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=3}} | ||
| + | <noinclude><translate><!--T:38--> | ||
| + | </noinclude> | ||
| + | <!-- 3. Discuss with a group the effects of water by flooding and erosion. --> | ||
| + | Flooding can cause extensive damage to land and property. It is also blamed for lose of life, serious injury, and damage to sewer systems and roads. Damage caused by a flood also have serious cost of repair associated with it. | ||
| − | + | <!--T:30--> | |
| + | Erosion can occur by means of water, causing soil and rock to be removed which can cause considerable damage. | ||
| − | ==4. Explain the following terms with reference to changes in water state. == <!--T: | + | <!--T:39--> |
| − | + | <noinclude></translate></noinclude> | |
| − | + | {{CloseReq}} <!-- 3 --> | |
| − | + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=4}} | |
| − | + | <noinclude><translate><!--T:40--> | |
| − | + | </noinclude> | |
| − | + | <!-- 4. Explain the following terms with reference to changes in water state. --> | |
| + | [[File:Aggregate phase.svg|thumb|400px|Phase transitions of water]] | ||
| + | <noinclude></translate></noinclude> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=4a}} | ||
| + | <noinclude><translate><!--T:41--> | ||
| + | </noinclude> | ||
| + | From gas (water vapor) to liquid. | ||
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 4a --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=4b}} | ||
| + | <noinclude><translate><!--T:42--> | ||
| + | </noinclude> | ||
| + | From liquid to gas (water vapor). | ||
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 4b --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=4c}} | ||
| + | <noinclude><translate><!--T:43--> | ||
| + | </noinclude> | ||
| + | From liquid to solid (ice). | ||
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 4c --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=4d}} | ||
| + | <noinclude><translate><!--T:44--> | ||
| + | </noinclude> | ||
| + | From solid to liquid. | ||
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 4d --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=4e}} | ||
| + | <noinclude><translate><!--T:45--> | ||
| + | </noinclude> | ||
| + | From gas directly to solid without becoming liquid. | ||
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 4e --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=4f}} | ||
| + | <noinclude><translate><!--T:46--> | ||
| + | </noinclude> | ||
| + | From solid directly to gas without becoming liquid. | ||
| + | {{clear}} | ||
| − | ==5. Be able to explain and illustrate making use of photographs, diagrams or sketches the following types of water: == <!--T: | + | <!--T:47--> |
| − | + | <noinclude></translate></noinclude> | |
| + | {{CloseReq}} <!-- 4f --> | ||
| + | {{CloseReq}} <!-- 4 --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=5}} | ||
| + | <noinclude><translate><!--T:48--> | ||
| + | </noinclude> | ||
| + | <!-- 5. Be able to explain and illustrate making use of photographs, diagrams or sketches the following types of water: --> | ||
| + | <noinclude></translate></noinclude> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=5a}} | ||
| + | <noinclude><translate><!--T:49--> | ||
| + | </noinclude> | ||
| + | Filtered water is water that has gone through a process of being cleaned by having it flow through fine strainer. | ||
| − | == | + | <!--T:50--> |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 5a --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=5b}} <!--T:7--> | ||
| + | <noinclude><translate><!--T:51--> | ||
| + | </noinclude> | ||
| + | Soft water is water that has gone through a process of having calcium, magnesium, along with other chemicals removed that reside in hard water. | ||
| − | == | + | <!--T:52--> |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 5b --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=5c}} <!--T:8--> | ||
| + | <noinclude><translate><!--T:53--> | ||
| + | </noinclude> | ||
| + | Distilled water is water that has first been boiled into steam in order to remove the impurities. Through condensation, the steam turned back into pure water into a clean container. | ||
| − | == | + | <!--T:54--> |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 5c --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=5d}} <!--T:9--> | ||
| + | <noinclude><translate><!--T:55--> | ||
| + | </noinclude> | ||
| + | Rain water is water vapor that became heavy enough to descend under the force gravity. | ||
| − | == | + | <!--T:56--> |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 5d --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=5e}} <!--T:10--> | ||
| + | <noinclude><translate><!--T:57--> | ||
| + | </noinclude> | ||
| + | Snow water is rain water that comes through the atmosphere at freezing temperatures below 32 °F or 0.0 °C. | ||
| − | + | <!--T:58--> | |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 5e --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=5f}} <!--T:11--> | ||
| + | <noinclude><translate><!--T:59--> | ||
| + | </noinclude> | ||
| + | De-ionized Water is water that has gone through a purification process where salts have been removed; such as sodium, calcium, chloride, and bromide. | ||
| − | == | + | <!--T:60--> |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 5f --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=5g}} <!--T:12--> | ||
| + | <noinclude><translate><!--T:61--> | ||
| + | </noinclude> | ||
| + | Raw Water is considered water that has not gone through any type of purification process. This type of water is found in lakes, rivers, and rain water containing millions of germs and viruses. | ||
| − | == | + | <!--T:62--> |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 5g --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=5h}} <!--T:13--> | ||
| + | <noinclude><translate><!--T:63--> | ||
| + | </noinclude> | ||
| + | Hard Water is water that is saturated with minerals such as calcium, iron, magnesium, and many other inorganic minerals. Water in lakes, rivers, and well water is considered hard water. | ||
| + | <!--T:64--> | ||
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 5h --> | ||
| + | {{CloseReq}} <!-- 5 --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=6}} | ||
| + | <noinclude><translate><!--T:65--> | ||
| + | </noinclude> | ||
| + | <!-- 6. What is a watershed? Discuss with a group the importance of watersheds. --> | ||
| + | A watershed is a considerably large area of land where rainfall or melted snow is gathered at a lower elevation point. | ||
| − | + | <!--T:31--> | |
| + | A group discussion with Pathfinders and staff should be conducted to understand the importance of watersheds. Selected topics of interest could include their protection and the role they play with regard to our water supply. | ||
| − | + | <!--T:32--> | |
| + | Note that ground pollution is generally picked up by water as it flows downhill, so any pollution within the watershed will be concentrated at the lower elevation. Since watersheds cover such vast areas, they can pick up an enormous amount of pollutants and concentrate them in a much smaller area. | ||
| − | == | + | <!--T:66--> |
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 6 --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=7}} | ||
| + | <noinclude><translate><!--T:67--> | ||
| + | </noinclude> | ||
| + | <!-- 7. How does filtering water make it clean? --> | ||
| + | The process of filtering water removes impurities such as sediments, bacteria, protozoa, and microbial cysts (that can cause disease) from water by means of a fine physical barrier. Chemical and biological processes are also used to filter water. A filter is designed to cleanse water to certain levels, depending on what purpose the water will serve. Irrigation, drinking water, aquariums, and swimming pools are common applications of water filtering. | ||
| − | <!--T: | + | <!--T:68--> |
| − | + | <noinclude></translate></noinclude> | |
| + | {{CloseReq}} <!-- 7 --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=8}} | ||
| + | <noinclude><translate><!--T:69--> | ||
| + | </noinclude> | ||
| + | <!-- 8. On your own or with a group, perform the following experiment and explain in your results what can be done to keep clean water sources. --> | ||
| − | <!--T: | + | <!--T:70--> |
| − | + | <noinclude></translate></noinclude> | |
| + | {{CloseReq}} <!-- 8 --> | ||
| + | {{ansreq|page={{#titleparts:{{PAGENAME}}|2|1}}|num=9}} | ||
| + | <noinclude><translate><!--T:71--> | ||
| + | </noinclude> | ||
| + | <!-- 9. Develop a spiritual application from water and share it with your group. --> | ||
| − | <!--T: | + | <!--T:33--> |
| − | + | There are many angles to use in developing a spiritual application. Here are some ideas to get you going: | |
| − | + | *Jesus as the Living Water | |
| − | + | *Filtering pollutants vs preventing pollution as applied to sin. | |
| − | + | *The Earth as a watershed for sin when Satan and his angels were cast to the Earth. | |
| − | + | *The hydrosystem as created vs its present state after 6000 years of sin. | |
| − | + | *Distillation as an illustration of being born again. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | ||
| − | * | ||
| − | * | ||
| − | * | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | <!--T:72--> | ||
| + | <noinclude></translate></noinclude> | ||
| + | {{CloseReq}} <!-- 9 --> | ||
| + | <noinclude><translate></noinclude> | ||
==References== <!--T:28--> | ==References== <!--T:28--> | ||
<!--T:29--> | <!--T:29--> | ||
<noinclude></translate></noinclude> | <noinclude></translate></noinclude> | ||
| + | {{CloseHonorPage}} | ||
Latest revision as of 19:06, 14 September 2021
Skill Level
2
Year
2015
Version
07.02.2026
Approval authority
North American Division
1
For tips and instruction see Water Science.
2
- Solid (ice)
- reflects the sun’s heat, thus keeping the earth cool by limiting the amount of light that is turned into heat. Ice also helps to control the temperature of the earth by being a sink for heat.
- Liquid (water)
- has a natural ability to absorb carbon dioxide from the atmosphere. This process allows the temperature of the earth to come down. As the sun shines on water, the absorbed light increases the temperature of the water. This process of heating and cooling allows for water to maintain a stable temperature.
- Gas (vapor)
- plays a critical role in maintain earth’s weather. The process of water evaporation allows for the formation of clouds. As the clouds become heavy, they produce rain, which cleans the air and waters vegetation.
3
Flooding can cause extensive damage to land and property. It is also blamed for lose of life, serious injury, and damage to sewer systems and roads. Damage caused by a flood also have serious cost of repair associated with it.
Erosion can occur by means of water, causing soil and rock to be removed which can cause considerable damage.
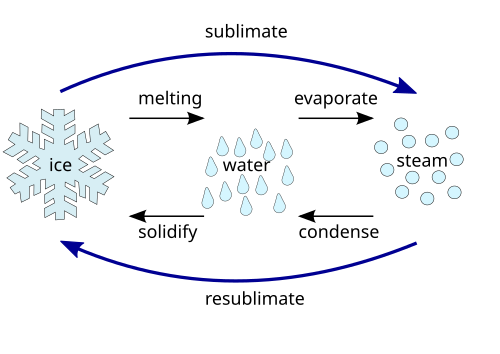
4
4a
From gas (water vapor) to liquid.
4b
From liquid to gas (water vapor).
4c
From liquid to solid (ice).
4d
From solid to liquid.
4e
From gas directly to solid without becoming liquid.
4f
From solid directly to gas without becoming liquid.
5
5a
Filtered water is water that has gone through a process of being cleaned by having it flow through fine strainer.
5b
Soft water is water that has gone through a process of having calcium, magnesium, along with other chemicals removed that reside in hard water.
5c
Distilled water is water that has first been boiled into steam in order to remove the impurities. Through condensation, the steam turned back into pure water into a clean container.
5d
Rain water is water vapor that became heavy enough to descend under the force gravity.
5e
Snow water is rain water that comes through the atmosphere at freezing temperatures below 32 °F or 0.0 °C.
5f
De-ionized Water is water that has gone through a purification process where salts have been removed; such as sodium, calcium, chloride, and bromide.
5g
Raw Water is considered water that has not gone through any type of purification process. This type of water is found in lakes, rivers, and rain water containing millions of germs and viruses.
5h
Hard Water is water that is saturated with minerals such as calcium, iron, magnesium, and many other inorganic minerals. Water in lakes, rivers, and well water is considered hard water.
6
A watershed is a considerably large area of land where rainfall or melted snow is gathered at a lower elevation point.
A group discussion with Pathfinders and staff should be conducted to understand the importance of watersheds. Selected topics of interest could include their protection and the role they play with regard to our water supply.
Note that ground pollution is generally picked up by water as it flows downhill, so any pollution within the watershed will be concentrated at the lower elevation. Since watersheds cover such vast areas, they can pick up an enormous amount of pollutants and concentrate them in a much smaller area.
7
The process of filtering water removes impurities such as sediments, bacteria, protozoa, and microbial cysts (that can cause disease) from water by means of a fine physical barrier. Chemical and biological processes are also used to filter water. A filter is designed to cleanse water to certain levels, depending on what purpose the water will serve. Irrigation, drinking water, aquariums, and swimming pools are common applications of water filtering.
8
Investigating Pollution
There are many ways that water can become polluted. Aside from natural pollution such as soil, leaves and living organisms, people cause the most serious pollution. From agricultural fertilizers and pesticides to urban runoff and industrial waste, pollutants can seep into groundwater that is often used as a source for drinking water. Follow the instructions in this experiment to make polluted water and observe what pollutants may do to water supplies.
Materials:
- 8 one-pint jars(four with tight-fitting lids)
- Masking tape
- Funnel
- Cotton
- Motor Oil
- Vinegar
- Laundry detergent
- Soil
- Plastic Cups
Procedure:
- Label two sets of jars. Number four of the jars (1,2,3 and 4) with masking tape. Make sure these four jars have lids that will fit tightly. Fill this set of jars half full of water. Number the other four jars (5,6,7 and 8) with masking tape and set them aside.
- Observe the water in jar #1. Record your observations.
- Put one tablespoon of motor oil in jar #2. Tighten the lid and shake the jar carefully. Record your observations.
- Put a tablespoon of vinegar in jar #3. Tighten the lid and shake the jar carefully. Record your observations.
- Put a tablespoon of detergent in jar #4. Tighten the lid and shake the jar carefully. Record your observations.
- Place a piece of cotton in the funnel and then add some soil. Place the funnel on empty jar #5.
- Pour the contents of jar #1 (water only) into the funnel and let it drip through the funnel into jar #5.
- Move the funnel with the cotton and soil to empty jar #6. Pour the contents of jar #2 (oil and water) into the funnel and let it drip through the funnel into jar #6. Observe and record your observations.
- Move the funnel with the cotton and soil to empty jar #7. Pour the contents of jar #3 (vinegar) into the funnel and let it drip through the funnel into jar #7. Observe and record your observations.
- Move the funnel with the cotton and soil to empty jar #8. Pour the contents of jar #4 (detergent) into the funnel and let it drip through the funnel into jar #8. Record your observations.
Making Discoveries:
If these substances were added to a real water source, how might they affect the water?
How might animals and people be affected?
Can you think of instances where materials such as these (oil, chemical detergent, etc.) might have been spilled or dumped and possibly endangered a water supply?
What measures might a community take to prevent such accidents?
9
There are many angles to use in developing a spiritual application. Here are some ideas to get you going:
- Jesus as the Living Water
- Filtering pollutants vs preventing pollution as applied to sin.
- The Earth as a watershed for sin when Satan and his angels were cast to the Earth.
- The hydrosystem as created vs its present state after 6000 years of sin.
- Distillation as an illustration of being born again.